Figure 1.
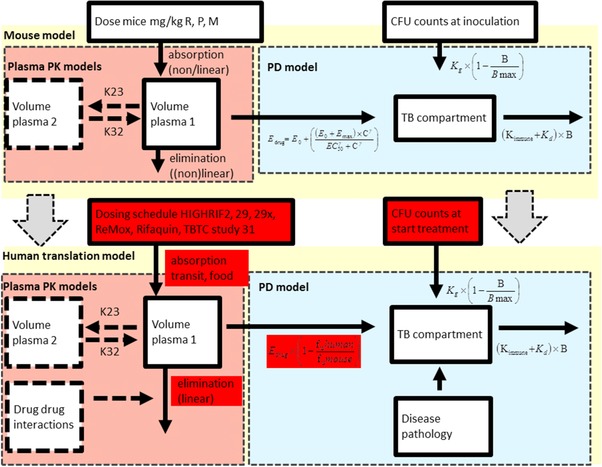
A translational pharmacokinetic/pharmacodynamic (PK/PD) model derived from mouse data used to predict colony forming unit (CFU) counts in patients. In this translational model, we assumed the following characteristics: (i) that the rate of bacterial growth in BALB/c mice and in human patients with drug‐sensitive pulmonary tuberculosis (TB) are the same, and (ii) that the concentration‐response relationship in mice and human patients at the site of action is the same. Therefore, the parameters assumed to be equivalent to the preclinical values are not highlighted, whereas the patient‐based parameters are highlighted in red. Baseline, number of bacteria at inoculation; Bmax, maximum number of bacteria; γ, the sigmoidicity factor, which defines the shape of the relationship; γimmune response, the sigmoidicity factor, which defines the shape of the immune system – bacterial effect relationship; IT50, the time that produces 50% of the maximum immune effect; Kdeath, bacterial death constant; Kgrowth, bacterial growth constant; M, moxifloxacin; P, rifapentine; R, rifampin; θKDOI.0, immune killing rate in treated animals at average incubation time; θKDOI.t, the increase in killing rate in experiments with a longer than average incubation period; θKIND, the maximum immune dependent killing rate in untreated animals; EC50, the antibiotic concentration that produces 50% of the maximum effect; Edrug, effect with a certain drug treatment; Emax, the maximal achievable effect with a certain drug treatment.
