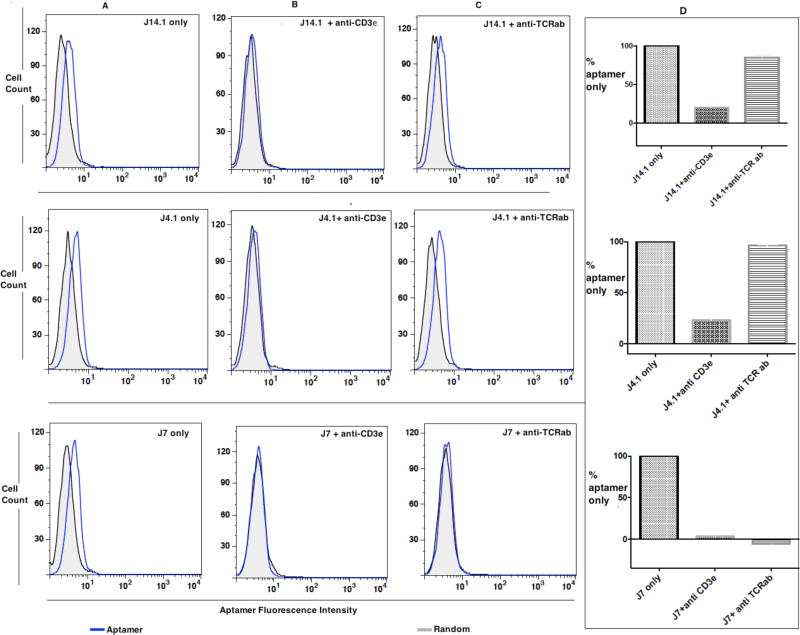
Figure 4.
Epitope identity. Flow cytometric competitive binding analysis of J4, J7 and J14.1 without (A) or with anti-CD3ε (B) or with anti-TCRαβ antibody (C) and overall conclusion from data presented in A, B and C from two independent experiments normalized to the aptamer binding to Jurkat.E6 cells with no added antibody (D). Each FITC-labeled random control or J4.1, J7 or J14.1 (1 µM) was incubated for 40 minutes on ice with 75 × 103 Jurkat.E6 cells. Then, binding buffer or anti-CD3ε HIT3a clone (panel B) or anti-TCRαβ antibody (panel C) was added and incubated for an additional 40 minutes. The cells were washed with 1.5 mL of wash buffer and the binding of respective aptamer analyzed by flow cytometry. Aptamer fluorescence intensity on X-axis indicates the binding of each aptamer. Thus, increment of fluorescence intensity can be directly compared to baseline random control as an indicator of aptamer binding. Aptamer fluorescence intensity on the X-axis shifted to background when anti-CD3ε HIT3a was added to all three aptamers pre-incubated with Jurkat.E6 cells, and binding of J7 and J14.1 was affected when anti-TCRαβ antibody was added. On the other hand, no difference in fluorescence intensity was observed for random control (gray-black); therefore, competitive binding experiments against aptamer J7, J4.1 and J14.1 using anti- CD3ε antibody showed that the stronger binder to CD3ε, effectively displaces all three aptamers. Binding of corresponding antibody is shown in Figure S5 in Supporting Information.