Figure 2. HCV core protein increases the level of phosphorylated 4E-BP1.
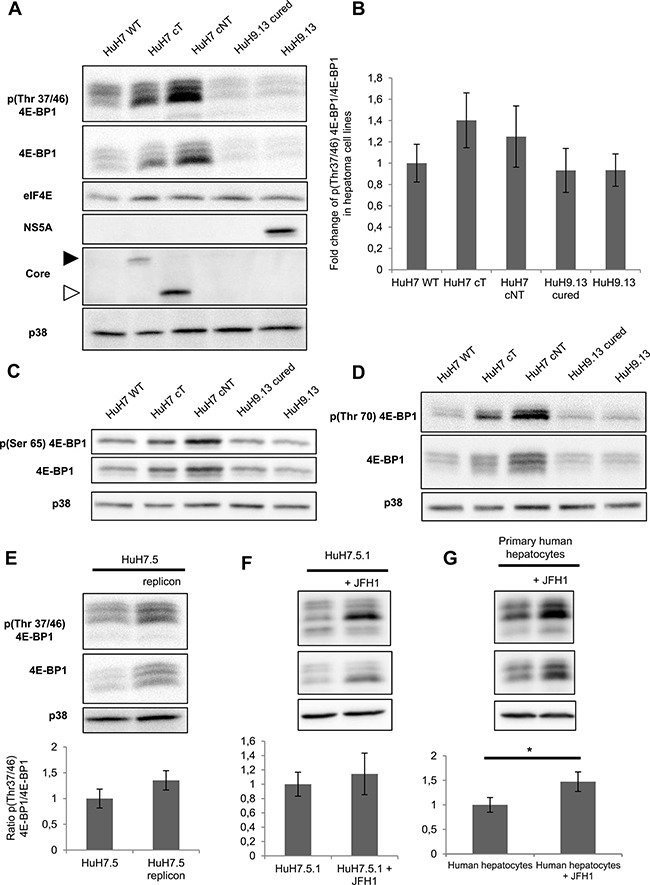
(A) Immunoblotting analysis of 4E-BP1 phosphorylation on Thr 37/46 in lysates of HuH7 controls (WT), HuH7 stably expressing core protein variants either isolated from tumor or non-tumor cirrhotic (cT or cNT) areas and HuH9.13 harboring the HCV NS3-NS5B subgenomic replicon and its related control (HuH9.13 cured). HCV NS5A and core expressions are shown. When the membrane was probed with an antibody directed against core antibody, an expected signal at 20 kDa was obtained in HuH7 cNT (white arrow), however this signal was observed at 25 kDa in HuH7 cT since they express a FLAG-tagged core (black arrow). (B) Graphic representation of average fold changes of phospho Thr37/46 4E-BP1 normalized to 4E-BP1 (C, D) Protein lysates of HuH7 WT, cT, cNT, HuH9.13 cured and HuH9.13 were analyzed by Western blotting for the phosphorylation state of 4E-BP1 at Ser 65 and Thr 70, respectively. (E) Immunoblotting analysis of 4E-BP1 phosphorylation at Thr 37/46 and the relative ratios of p4E-BP1/4E-BP1 in HuH7.5 replicon, harboring all HCV proteins. (F, G) Protein extracts of HuH7.5.1 and primary human hepatocytes infected or not with the HCV strain JFH1 were immubloted with phospho Thr37/46 4E-BP1. Fold changes of p4E-BP1 over 4E-BP1 are shown, *p value < 0.05. One representative immunoblot out of three independent experiments is shown and p38 was used as loading control.
