Figure 2. Aurora A is acetylated by ARD1.
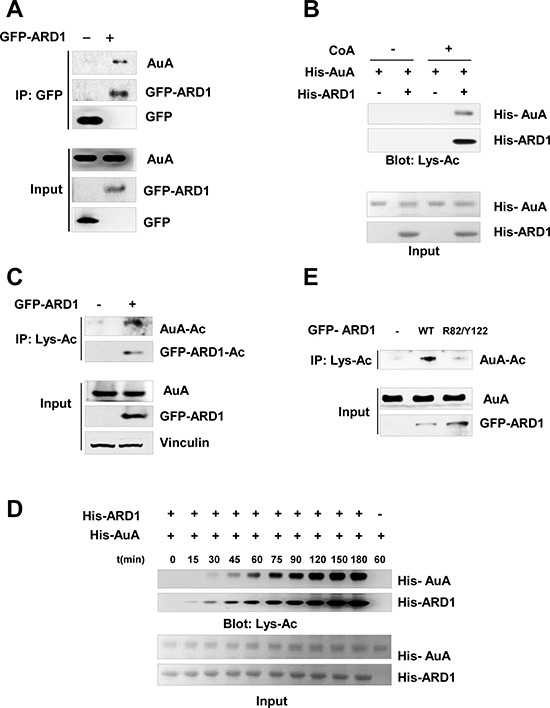
(A) AuA interacts with ARD1. Lysates from HEK293T cells overexpressing GFP-ARD1 were immunoprecipitated with anti-GFP antibody and immunoblotted with anti-AuA antibody or anti-GFP antibody. The experiments were performed at least three times independently. (B) AuA is acetylated by ARD1 in vitro. His-ARD1, His-AuA recombinants were subjected to in vitro acetylation assays with or without presence of acetyl group donor acetyl- coenzyme A (CoA) for 1 h, and acetylation levels of recombinants were assessed by western blotting using an anti-acetylated lysine antibody (Lys-Ac). Ponceau S staining shows the quantification of the input proteins. The experiments were performed at least three times independently. (C) Acetylated AuA level increases in GFP-ARD1 overexpressing cells. Lysates from GFP-ARD1 overexpressing MCF7 cells were immuprecipitated with anti-Lys-Ac antibody and analyzed by immunoblotting with anti-AuA antibody or anti-GFP antibody. The experiments were performed at least three times independently. (D) AuA acetylation occurs in a time-dependent manner. His-ARD1 recombinants were subjected to in vitro acetylation assays for series of time, and acetylation levels of recombinants were assessed by western blotting using an anti-Lys-Ac antibody. Quantification of the input proteins were analyzed by Ponceau S staining. The experiments were performed at least three times independently. (E) AuA acetylation is dependent on ARD1 acetyltransferase activity. MCF7 cells were transfected with wild type (WT) GFP-ARD1 or GFP-ARD1 R82F/Y122A mutant. The extracts from the overexpressing cells were immoprecipitated with anti Lys-Ac antibody and acetylated AuA levels were analyzed by immunoblotting with anti-AuA antibody. The experiments were performed at least three times independently.
