Abstract
Background
It’s difficult to diagnose gastroesophageal anastomotic leakage (GAL) at early postoperative stage. This study was conducted to evaluate the early predictive value of plasma cytokines levels on GAL in patients undergoing esophagectomy.
Methods
Consecutive esophageal cancer patients who underwent esophagectomy and admitted to Surgical Intensive Care Unit (SICU) just after surgery were retrospectively analyzed. The baseline and postoperative 1 day plasma cytokine levels were collected and analyzed to evaluate the predictive value for clinically important anastomotic leakage. Area under receiver operating characteristic curve (AUROC) analysis was also performed.
Results
A total of 183 patients were included. Sixteen patients (8.74%) experienced GAL (GAL group) and the others did not (non-GAL group). The concentrations of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-2R, IL-6, IL-8 and IL-10 in plasma on the first postoperative day significantly increased in the GAL group than in the non-GAL group (P<0.05). IL-6, IL-8 and IL-10 were fair predictors of GAL (AUROC >0.7) and the other two cytokines were poorly predictive (AUROC <0.7). The mean length of ICU and hospital stay were significantly longer in the GAL group than in the non-GAL group (P<0.05).
Conclusions
Plasma concentrations of IL-6, IL-8 and IL-10 on the first postoperative day can predict clinically important GAL in patients undergoing esophagectomy.
Keywords: Esophageal cancer, anastomotic leakage, interleukin (IL), tumor necrosis factor
Introduction
The incidence of esophageal carcinoma is rapidly increasing and surgical resection remains the standard therapy. Gastroesophageal anastomotic leakage (GAL) is universally known as an important cause of postoperative morbidity and mortality of esophageal carcinoma, with the incidence ranging from 5% to up to 20% (1,2). Prediction of GAL is an important aspect of perioperative management. Several pre- and intra-operative risk factors of GAL have been identified such as age, male gender, emergency surgery, smoking, alcohol abuse, American Society of Anesthesiologists (ASA) score, obesity, low serum albumin levels, diabetes, hypertension, renal failure and cardiovascular disease (3-5). However, the specificity of these indicators is not very high. There were few researches focusing on early postoperative predictor of GAL in esophagectomy patients. Sparse researches indicated that C-reactive protein (CRP) level on postoperative day 3 was a reliable predictor of anastomotic leakage after esophagectomy (6,7). But CRP is a kind of acute phrase reactive protein which is nonspecifically involved in systemic or local inflammatory responses. In most clinical situations, early postoperative diagnosis of GAL is very difficult.
Some studies reported that in patients underwent colorectal surgery, peritoneal cytokine levels could predict anastomotic leakage at a preclinical stage, even as early as the first postoperative day (8,9). Plasma level of interleukin (IL)-6 was also higher on day 1 after colorectal surgery in anastomotic leak patients (8). Colon and esophagus are different parts of the digestive tract, which may have similar clinical and pathophysiological changes in postoperative anastomotic leakage patients. This study was conducted to evaluate the early predictive value of plasma cytokines on GAL in patients underwent esophagectomy.
Methods
Participants
This was a single-center retrospective study based on data from collected database and medical records. From July 2015 to June 2016, consecutive esophageal carcinoma patients treated with esophagectomy and admitted to Surgical Intensive Care Unit (SICU) of Zhongshan Hospital Fudan University just after the surgery were enrolled. Exclusion criteria including: (I) age <18 years; (II) emergency surgeries; (III) current use of steroid; (IV) ASA ≥4. This study was approved by the Review Board of the Ethics Committee of Zhongshan Hospital Fudan University, and was in compliance with the institution’s requirements.
Data collection
In our center, cytokine levels were routinely tested before and on the first postoperative day among esophageal cancer patients in order to reflect the degree of inflammatory response. The following clinical, physiological and outcome data were collected: age, gender, personal history, preoperative albumin, operative-related information (including surgical approach, tumor position, anastomotic position, mean operation time and histology), pre- and postoperative 1 day plasma cytokines [including tumor necrosis factor-alpha (TNF-α), IL-2 receptor (IL-2R), IL-6, IL-8 and IL-10], length of stay in ICU and hospital, 30-day and in-hospital mortality.
Surgery and postoperative assessment
All the operations were performed under combined general-epidural anesthesia by experienced thoracic surgeons. After operation, all patients were immediately transferred to SICU. Water-soluble contrast swallow was done on the 3rd to 5th postoperative day before starting oral intake. GAL was diagnosed when one of the three following conditions was met: (I) chest radiography or computerized tomography obtained the presence of intra-thoracic collection of swallowing contrast agent adjacent to the anastomosis; (II) extravasation of gastrointestinal tract content through a wound or drainage tube; (III) direct observation of GAL by postoperative gastroscopy examination; (IV) intraoperative diagnosis. If GAL was diagnosed, the patient mainly treated with drainage, antibiotics, nutritional support or reoperation if it was clinically important.
Statistical analysis
Statistical analysis was performed with SPSS version 21 (IBM Corporation, Armonk, NY, USA). Continuous variable parametricity was tested using the Shapiro-Wilk test. Parametric continuous variables were presented as mean ± standard deviation and analyzed with the t-test, while nonparametric continuous variables were analyzed with the Mann-Whitney U test. Categorical variables were analyzed with the χ2 test or Fisher exact test. Area under receiver operating characteristic curve (AUROC) analysis was calculated to determine the predictive value of the plasma cytokines for GAL. AUROC values were interpreted as follows: <0.70 = poor; 0.70 to 0.80 = fair; 0.80 to 0.90 = good; >0.90 = excellent. The optimal cutoff value was calculated by the Youden index. A P value of <0.05 (2-sided) was considered to be statistically significant.
Results
Baseline patient characteristics
A total of 183 esophageal cancer patients (male/female: 104/79) with the average age of 64.8±5.0 years old were enrolled. Among these patients, a total of 16 (8.74%) experienced an anastomotic leakage (GAL group) while the other 167 did not (non-GAL group). There was no significant difference in baseline characteristics between these two groups and the demographic data was detailed in Table 1. Of these patients who experienced GAL, 3 required reoperation (18.75%), 9 required percutaneous drainage (56.25%) and 4 required intravenous antibiotics only (25%).
Table 1. Baseline characteristics.
| Characteristics | Non-GAL group (n=167) | GAL group (n=16) | P |
|---|---|---|---|
| Median age, year [range] | 63 [49–78] | 65 [51–80] | 0.867 |
| Gender, n (%) | 0.566 | ||
| Male | 94 (56%) | 10 (63%) | |
| Female | 73 (44%) | 6 (37%) | |
| BMI (SD) | 20.9 (2.6) | 20.1 (1.8) | 0.387 |
| Diabetes, n (%) | 52 (31%) | 4 (40%) | 0.490 |
| ASA score, n (%) | 0.321 | ||
| I | 61 (36%) | 5 (31%) | |
| II | 88 (53%) | 8 (50%) | |
| III | 18 (11%) | 3 (19%) | |
| Albumin, g/L (SD) | 40.8 (3.0) | 41.1 (3.0) | 0.682 |
| Neoadjuvant chemoradiotherapy, n (%) | 45 (27%) | 5 (31%) | 0.742 |
| Mean operation time, min (SD) | 298.1 (105.6) | 323.5 (132.1) | 0.586 |
| Surgical procedure, n (%) | 0.782 | ||
| Sweet esophagectomy | 35 (21%) | 3 (19%) | |
| McKeown esophagectomy | 85 (51%) | 8 (50%) | |
| Ivor-Lewis esophagectomy | 47 (28%) | 5 (31%) | |
| Tumor position, n (%) | 0.479 | ||
| Upper thoracic | 25 (15%) | 1 (6%) | |
| Middle thoracic | 89 (53%) | 9 (56%) | |
| Lower thoracic | 41 (25%) | 4 (25%) | |
| Gastroesophageal junction | 12 (7%) | 2 (13%) | |
| Mode selection, n (%) | 0.537 | ||
| VATS | 112 (67%) | 11 (69%) | |
| Thoracotomy | 55 (33%) | 5 (31%) | |
| Histology, n (%) | 0.741 | ||
| Squamous carcinoma | 125 (75%) | 13 (81%) | |
| Adenocarcinoma | 42 (25%) | 3 (19%) | |
| AJCC stage, n (%) | 0.365 | ||
| I | 40 (24%) | 4 (25%) | |
| II | 86 (51%) | 9 (56%) | |
| III | 41 (25%) | 3 (19%) |
GAL, gastroesophageal anastomotic leakage; ASA, American Society of Anesthesiologists; BMI, body mass index; AJCC, American Joint Committee on Cancer.
Plasma concentrations of cytokines
Preoperative plasma concentrations of TNF-α, IL-2R, IL-6, IL-8 and IL-10 in both groups were parallel and in normal range (Table 2). On the first postoperative day, all these cytokines levels of the two groups obviously increased and each cytokine concentration in the GAL group were significantly higher than that in the non-GAL group (P<0.05) (Table 2).
Table 2. Preoperative and postoperative cytokines in the GAL & the non-GAL group.
| Variables | Non-GAL group (n=167) | GAL group (n=16) | P |
|---|---|---|---|
| Preoperative cytokine, pg/mL | |||
| TNF-α | 7.1±3.2 | 8.1±3.5 | 0.58 |
| IL-2R | 274.9±94.8 | 267.4±55.3 | 0.91 |
| IL-6 | 11.5±11.4 | 13.7±9.3 | 0.17 |
| IL-8 | 1.9±1.02 | 1.9±0.6 | 0.20 |
| IL-10 | 2.1±1.1 | 2.0±0.4 | 0.28 |
| POD1 cytokine, pg/mL | |||
| TNF-α | 11.4±7.8 | 28.0±31.2 | 0.032 |
| IL-2R | 642.0±252.4 | 1,730.8±613.1 | 0.021 |
| IL-6 | 101.8±88.7 | 292.7±152.8 | 0.008 |
| IL-8 | 31.2±29.0 | 544.1±657.8 | 0.009 |
| IL-10 | 8.1±7.5 | 61.7 ±49.8 | 0.006 |
POD, postoperative day; TNF-α, tumor necrosis factor alpha; IL-2R, interleukin 2 receptor; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10.
Predictive value of plasma cytokines
AUROC value of each cytokine was calculated and according to these values, IL-6, IL-8 and IL-10 were fair predictors of GAL (AUROC >0.7), while TNF-α and IL-2R were poor predictors (AUROC <0.7, Table 3 and Figure 1). Considering the biggest AUROC value, IL-10 was the best early postoperative predictor of GAL among these cytokines with the optimal cutoff value of 17.2 pg/mL, the sensitivity of 0.667 and the specificity of 0.848. The ROC curve analysis of all cytokines was shown in Table 3.
Table 3. Plasma cytokine ROC curve analysis.
| Cytokine | AUROC | Standard error | 95% CI | Optimal cutoff value (pg/mL) | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| TNF-α | 0.683 | 0.092 | 0.504–0.863 | 18.5 | 0.533 | 0.848 |
| IL-2R | 0.696 | 0.094 | 0.511–0.881 | 785.4 | 0.733 | 0.761 |
| IL-6 | 0.735 | 0.068 | 0.601–0.869 | 74.6 | 1.000 | 0.457 |
| IL-8 | 0.720 | 0.081 | 0.562–0.879 | 61.1 | 0.600 | 0.783 |
| IL-10 | 0.784 | 0.072 | 0.643–0.926 | 17.2 | 0.667 | 0.848 |
AUROC, area under receiver operating characteristic curve; TNF-α, tumor necrosis factor alpha; IL-2R, interleukin 2 receptor; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10.
Figure 1.
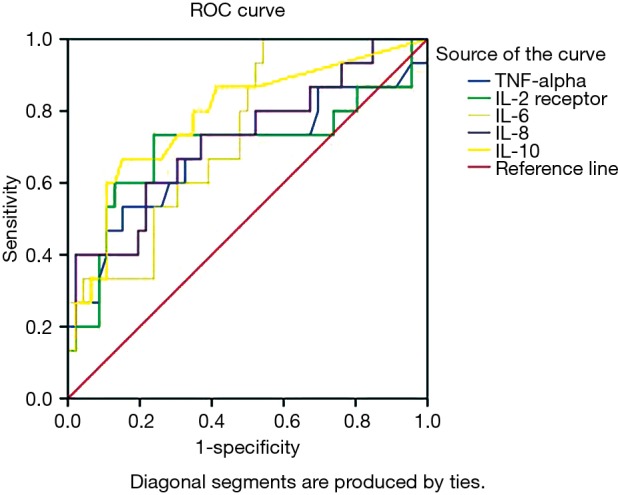
Plasma cytokine receiver operating characteristic curves. TNF-alpha, tumor necrosis factor alpha; IL-2R, interleukin 2 receptor; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10.
The mean length of stay in ICU and hospital were significantly longer in the GAL group than in the non-GAL group (2.8±2.5 vs. 9.2±6.8 d, P=0.008; 15.2±8.8 vs. 42.6±13.6 d, P<0.001). No patient died during the hospitalization period, therefore the 30-day and in-hospital mortality of both groups were 0%.
Discussion
To the best of our knowledge, this was the first study focused on the relationship between early postoperative plasma cytokines levels and the occurrence of GAL. In this study, we found that on the first postoperative day, plasma cytokines concentrations in the GAL group were significant higher than in the non-GAL group. Elevated plasma levels of IL-6, IL-8 and IL-10 were associated with higher incidence of GAL, and IL-10 appeared to be the best predictor based on the AUROC value.
GAL remains a serious complication after esophagectomy and the diagnosis relies on clinical presentation and imaging studies. The occurrence of GAL is a progressive process and the early presentation is often nonspecific and heterogeneous which may delay the diagnosis. Before systemic symptoms such as fever, increased heart rate and leukocytosis become severe, inflammatory response at the site of the anastomosis, involving the increasing of immune cells and cytokines, first takes place (10). Some cytokines such as TNF-α, IL-6 and IL-8 are pro-inflammatory cytokines mediating inflammatory response and IL-10 is considered as an anti-inflammatory cytokine. IL-6 is a pleiotropic cytokine that plays a major role in the response to injury or infection and also involved in immune response and many inflammatory disease (11-14). IL-8 is a famous neutrophil chemokine which induces re-arrangement of cytoskeleton, changes in intracellular Ca2+ levels, activation of integrins and exocytosis of granule proteins (15). The major role of IL-10 is to limit the extent of the activation of both the innate and the adaptive immune cells to maintain a homeostatic state. This role is vitally important in protecting the host from infection-associated immunopathology, autoimmunity, and allergy. Sammour et al. found that after colorectal surgery, peritoneal levels of IL-6 and IL-10 on the first postoperative day could predict clinically important anastomotic leakage (8). In the present study, the cytokines levels in the GAL group were significantly higher than in the non-GAL group. According to the AUROC value, IL-6, IL-8 and IL-10 were fair predictors of GAL and IL-10 appeared to be the best predictor. This result illustrated that in GAL patients, the systemic inflammatory response, both pro-inflammatory and anti-inflammatory process, were significantly enhanced at the early-stage after surgery which might indicate the leakage happened later.
TNF-α is a multifunctional cytokine that plays an important role in the pathogenesis of inflammatory and autoimmune diseases (16). Dysregulation of TNF-α has been implicated in a variety of human disease (17). IL-2 is primarily produced by activated T lymphocytes which exert biological effects through binding to the high affinity IL-2R (18). In the thymus where T cells become mature, IL-2 can prevent autoimmune diseases by promoting the differentiation of certain immature T cells into regulatory T cells and killing off other T cells which are prone to attack normal healthy cells. Sparreboom et al. indicated that the levels of TNF and IL-6 substantially increased in colorectal anastomotic leakage patients during the first postoperative days (19). In current study, significantly elevated plasma levels of TNF-α and IL-2R were obtained in the GAL group which might reflect the increasing trend of systemic inflammatory response in these patients. However, according to the AUROC value, these two cytokines were poor postoperative predictors of GAL.
For the purpose of determining a reference level of these cytokines, we used exact values rather than the comparative risk ratios in statistical analysis (9). Of the 5 plasma cytokines discussed in this study, IL-6, IL-8 and IL-10 appeared to be the better predictors which can objectively reflect the severity of inflammation caused by the procedure of anastomosis leakage. The systemic inflammatory response may indicate the subsequent occurrence of GAL. Based on the comprehensive analysis of these plasma cytokines, we can implement the corresponding treatment strategies at early time after the surgery so as to decrease the impact of the GAL to esophagectomy patients.
The incidence of GAL in this study was relatively high (20), but fortunately, the impact of GAL on these patients was not very severe. Only three patients needed reoperation and nobody died throughout the study. After fasting, nutritional support, drainage and antibiotic therapy, all the GAL patients recovered. We noted a significantly longer ICU and hospital stay in the GAL group which made us believe that early postoperative identification of patients at high risk of GAL could lower the damage caused by anastomotic leakage and reduce the length of ICU and hospital stay as well as the hospitalization costs.
There were several limitations in this study. First, it was a single-center retrospective trial conducted in SICU with limited number of subjects. Second, the surgeries were not performed by the same thoracic surgeon, which might bring about relevant bias. Third, there might be some missed GAL diagnosis cases because of the subtypical symptoms and image findings. So, the results must be interpreted carefully, and further well-designed prospective study based on larger population was required to confirm these findings.
In conclusion, plasma concentrations of TNF-α, IL-2R, IL-6, IL-8 and IL-10 on the first postoperative day after esophagectomy were significantly higher in GAL patients than in non-GAL patients, and IL-6, IL-8 and IL-10 levels can predict clinical important GAL.
Acknowledgements
None.
Ethical Statement: The study was approved by the Review Board of the Ethics Committee of Zhongshan Hospital Fudan University.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kassis ES, Kosinski AS, Ross P, Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. 10.1016/j.athoracsur.2013.07.119 [DOI] [PubMed] [Google Scholar]
- 2.Rutegård M, Lagergren P, Rouvelas I, et al. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol 2012;19:99-103. 10.1245/s10434-011-1926-6 [DOI] [PubMed] [Google Scholar]
- 3.Haga Y, Wada Y, Takeuchi H, et al. Prediction of anastomotic leak and its prognosis in digestive surgery. World J Surg 2011;35:716-22. 10.1007/s00268-010-0922-5 [DOI] [PubMed] [Google Scholar]
- 4.Aminian A, Panahi N, Mirsharifi R, et al. Predictors and outcome of cervical anastomotic leakage after esophageal cancer surgery. J Cancer Res Ther 2011;7:448-53. 10.4103/0973-1482.92016 [DOI] [PubMed] [Google Scholar]
- 5.van Rossum PS, Haverkamp L, Verkooijen HM, et al. Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophagealsurgery. Radiology 2015;274:124-32. 10.1148/radiol.14140410 [DOI] [PubMed] [Google Scholar]
- 6.Deitmar S, Anthoni C, Palmes D, et al. Are leukocytes and CRP early indicators for anastomotic leakage after esophageal resection? Zentralbl Chir 2009;134:83-9. 10.1055/s-0028-1098768 [DOI] [PubMed] [Google Scholar]
- 7.Edagawa E, Matsuda Y, Gyobu K, et al. C-reactive protein is a useful marker for early prediction of anastomotic leakage after esophageal reconstruction. Osaka City Med J 2015;61:53-61. [PubMed] [Google Scholar]
- 8.Sammour T, Singh PP, Zargar-Shoshtari K, et al. Peritoneal cytokine levels can predict anastomotic leak on the first postoperative day. Dis Colon Rectum 2016;59:551-6. 10.1097/DCR.0000000000000598 [DOI] [PubMed] [Google Scholar]
- 9.Cini C, Wolthuis A, D’Hoore A. Peritoneal fluid cytokines and matrix metalloproteinases as early markers of anastomotic leakage in colorectal anastomosis: a literature review and metaanalysis. Colorectal Dis 2013;15:1070-7. [DOI] [PubMed] [Google Scholar]
- 10.Trefzer U, Hofmann M, Sterry W, et al. Cytokine and anticytokine therapy in dermatology. Expert Opin Biol Ther 2003;3:733-43. 10.1517/14712598.3.5.733 [DOI] [PubMed] [Google Scholar]
- 11.Sehgal PB, Wang L, Rayanade R, et al. Interleukin-6-type cytokines. Ann NY Acad Sci 1995;762:1-14. 10.1111/j.1749-6632.1995.tb32309.x [DOI] [PubMed] [Google Scholar]
- 12.Gustot T, Lemmers A, Louis E, et al. Profile of soluble cytokine receptors in Crohn’s disease. Gut 2005;54:488-95. 10.1136/gut.2004.043554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto T, Saniabadi AR, Umegae S, et al. Impact of selective leukocytapheresis on mucosal inflammation and ulcerative colitis: cytokine profiles and endoscopic findings. Inflamm Bowel Dis 2006;12:719-26. 10.1097/00054725-200608000-00008 [DOI] [PubMed] [Google Scholar]
- 14.van der Poll T, van Deventer SJ. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin North Am 1999;13:413-26. 10.1016/S0891-5520(05)70083-0 [DOI] [PubMed] [Google Scholar]
- 15.Paccaud JP, Schifferli JA, Baggiolini M. NAP-1/IL-8 induces up-regulation of CR1 receptors in human neutrophil leukocytes. Biochem Biophys Res Commun 1990;166:187-92. 10.1016/0006-291X(90)91929-M [DOI] [PubMed] [Google Scholar]
- 16.Olszewski MB, Groot AJ, Dastych J, et al. TNF trafficking to human mast cell granules: mature chain-dependent endocytosis. J Immunol 2007;178:5701-9. 10.4049/jimmunol.178.9.5701 [DOI] [PubMed] [Google Scholar]
- 17.Brynskov J, Foegh P, Pedersen G, et al. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut 2002;51:37-43. 10.1136/gut.51.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malek TR. The biology of interleukin-2. Annu Rev Immunol 2008;26:453-79. 10.1146/annurev.immunol.26.021607.090357 [DOI] [PubMed] [Google Scholar]
- 19.Sparreboom CL, Wu Z, Dereci A, et al. Cytokines as early markers of colorectal anastomotic leakage: a systematic review and meta-analysis. Gastroenterol Res Pract 2016;2016:3786418. 10.1155/2016/3786418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Junemann-Ramirez M, Awan MY, Khan ZM, et al. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg 2005;27:3-7. 10.1016/j.ejcts.2004.09.018 [DOI] [PubMed] [Google Scholar]


