Abstract
The aim of the present study was to investigate the prognostic value of the combined platelet (PLT), fibrinogen (FBG), neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) (CO-NPF) for postoperative outcomes in patients with lung adenosquamous cancer (ASC). Test results from patients who presented at The Cancer Institute and Hospital of Tianjin Medical University between January 2005 and December 2013 were retrospectively reviewed. CO-NPF was scored between 0 and 4 according to increased PLT, FBG, NLR and PLR prior to being split into two groups based on the presence (≥2) or absence (<2) of the combination of increased inflammatory indexes. In total, data from 134 patients with ASC were reviewed for the present study. Multivariate analysis identified that increased CO-NPF (P=0.001 and P<0.001, respectively), PLR (P=0.011 and P=0.001, respectively) and FBG (P=0.001 and P<0.001, respectively) were independently associated with shorter disease-free survival (DFS) and overall survival (OS). NLR (P=0.006) and PLT (P=0.001) were independent prognostic factors for OS. The area under the receiver operating characteristic curves of CO-NPF (area under the curve, 0.652, P=0.008, 95% confidence interval, 0.551–0.752) was increased compared with NLR, PLR, PLT and FBG individually, suggesting that CO-NPF has greater predictive value. CO-NPF was significantly and independently associated with shorter DFS and OS, and had greater predictive value compared with NLR, PLR, PLT and FBG in patients with ASC who underwent surgery.
Keywords: lung adenosquamous cancer, neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, fibrinogen, platelet
Introduction
Lung cancer is a major health problem throughout the world, particularly in China (1,2). Lung adenosquamous cancer (ASC) is a subtype of non-small cell lung cancer (NSCLC) that accounts for between 0.4 and 4% of all lung malignancies (3–5). ASC is defined as a carcinoma containing squamous cell carcinoma (SCC) and adenocarcinoma (ADC) components with each comprising ≥10% of the total tumor (6). Although the symptoms of ASC are similar to those of other histological subtypes of NSCLC (7), aggressive characteristics including early metastasis, invasiveness, rapid growth and poor prognosis have been recognized (8–10). Consequently, valuable prognostic factors are urgently needed for patients with ASC.
The association between inflammation and cancer proposed by Virchow in 1863 is now widely accepted (11). Inflammation contributes to the survival, proliferation and metastasis of cancer cells, as well as promoting angiogenesis. In addition, inflammation prevents the adaptive immune response and is able to alter responses to systemic therapies (11,12). Numerous studies have revealed the association between inflammatory indexes and prognoses of patients with NSCLC, including platelet (PLT), fibrinogen (FBG), neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) (13–23). The study conducted by Unal et al (23) first identified the association between PLR and prognosis in patients with NSCLC, and suggested that assessing NLR and PLR together may predict the prognosis of patients with NSCLC. However, to the best of our knowledge, the prognostic value of these inflammatory markers for patients with ASC who have undergone surgery has not been reported. Therefore, the aim of the present study was to assess the association between NLR, PLR, PLT and FBG with the prognosis of patients with ASC and further analyze whether the combination of increased NLR, PLR, FBG and PLT (CO-NPF) was superior to any ratio alone.
Patients and methods
Study population
Patients who presented at The Cancer Institute and Hospital of Tianjin Medical University between January 2005 and December 2013 were enrolled in the present study. Demographic and clinicopathological characteristics as well as preoperative laboratory parameters were recorded and retrospectively reviewed. The study was supported by the Ethics Committee and the Institutional Review Board of The Cancer Institute and Hospital of Tianjin Medical University. Prior to enrollment into the present study, written informed consent was received from all patients or their families. The inclusion criteria were as follows: i) Histologically diagnosed with ASC; ii) with stage I–IIIA [7th Tumor-Node-Metastasis Classification of Malignant Tumors (TNM) (24)]; and iii) underwent surgery. Patients were excluded who met any of the following criteria: i) Presented with other types of carcinoma within 5 years; ii) succumbed within 1 month of surgery; iii) received adjuvant chemotherapy or radiotherapy prior to surgery; iv) suffered from active infection(s) prior to surgery; and v) lost to follow up. A total of 134 patients with ASC were recruited into the present study.
Definition of CO-NPF
NLR and PLR were defined as the ratio of neutrophils to lymphocytes and platelets to lymphocytes, respectively. According to threshold values that were determined by receiver operating characteristic (ROC) curve analysis, the NLR, PLR, PLT and FBG were each divided into two groups: <2.16 and ≥2.16, <145 and ≥145, <289 and ≥289 and <4 and ≥4, respectively. CO-NPF was defined as the combination of increased NLR, PLR, PLT and FBG. CO-NPF were scored between 0 and 4 according to the number of increased parameters (NLR, PLR, PLT and FBG). For example, patients with four increased indexes were scored 4, whereas patients with three, two, one or zero increased indexes were scored 3, 2, 1 or 0, respectively. CO-NPF was then divided into two groups according to the presence (≥2) or absence (<2) of the combination of increased inflammatory indexes.
Statistical analysis
Statistical analysis was performed using SPSS (version 22.0; IBM Corp., Armonk, NY, USA). Continuous data are presented as the mean ± standard deviation or median (range), and categorical data are expressed as percentages and frequencies. Differences between two groups were compared using χ2 test or Fisher's exact test. Overall survival (OS) was defined as the period from the date of surgery to the date of mortality or censoring. Disease-free survival (DFS) was calculated from the date of surgery to the date of relapse or mortality. Kaplan-Meier estimator survival curves were created and differences between survival curves were analyzed using the log-rank test. Independent prognostic factors were identified using a multivariate Cox's regression model in which all the significant variables that were identified by univariate analysis were involved in a forward conditional manner. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were obtained from multivariate analysis. Areas under ROC curves (AUCs) were applied to compare NLR, PLR, PLT, FBG and CO-NPF. P<0.05 was considered to indicate a statistically significant difference.
Results
In total, 134 patients with ASC were enrolled in the present retrospective study. For total patients, the 3-year and 5-year survival rates were 36.1 and 24.8%, respectively. The median follow-up time was 22 months (range, 2–120 months) and during the follow-up period 84 (61.8%) patients developed tumor relapse and 96 (70.6%) patients succumbed. Patient baseline characteristics are summarized in Table I. The median patient age was 60 years (range, 34–83 years). Of the patient cohort, 81 (60.5%) were male and 53 (39.5%) were female. The majority of patients were stage I–II (n=80, 59.7%), with 54 (40.3%) patients in stage IIIA. The majority of patients underwent pulmonary lobectomy (n=112, 83.6%), with 12 (8.9%) receiving local tumor resection and 10 (7.5%) receiving pneumonectomy. Of the total of 134 patients, 78 (58.2%) received platinum-based chemotherapy following surgery.
Table I.
Baseline patient characteristics.
| Characteristic | Number of patients (of a total of 134) (%) |
|---|---|
| Age, years | |
| Median (range), 60 (34–83) | |
| ≥60 | 60 (44.8) |
| <60 | 74 (55.2) |
| Sex | |
| Male | 81 (60.5) |
| Female | 53 (39.5) |
| Smoking index | |
| ≥400 | 60 (44.8) |
| <400 | 74 (55.2) |
| Lymph node metastasis | |
| Yes | 79 (59.0) |
| No | 55 (41.0) |
| TNM stage | |
| I–II | 83 (61.9) |
| IIIA | 51 (38.1) |
| Operative approach | |
| Local tumor resection | 12 (8.9) |
| Pulmonary lobectomy | 112 (83.6) |
| Pneumonectomy | 10 (7.5) |
| Albumin, g/dl | |
| Mean ± SD, 43.56±5.33 | |
| ≥49.5 | 10 (7.5) |
| <49.5 | 124 (92.5) |
| WBC count, ×109 cells/l | |
| Mean ± SD, 6.98±2.94 | |
| ≥5.6 | 99 (73.9) |
| <5.6 | 35 (26.1) |
| Neutrophil count, ×109 cells/l | |
| Mean ± SD, 4.23±1.61 | |
| Lymphocyte count, ×109 cells/l | |
| Mean ± SD, 1.96±0.65 | |
| NLR | |
| Mean ± SD, 2.36±1.13 | |
| ≥2.16 | 60 (44.8) |
| <2.16 | 74 (55.2) |
| PLR | |
| Mean ± SD, 143.14±65.00 | |
| ≥145 | 90 (67.2) |
| <145 | 44 (32.8) |
| PLT count, ×109 cells/l | |
| Mean ± SD, 254.12±72.30 | |
| ≥289 | 36 (26.9) |
| <289 | 98 (73.1) |
| FBG, g/l | |
| Mean ± SD, 3.64±0.89 | |
| ≥4 | 45 (33.6) |
| <4 | 89 (66.4) |
| CO-NPF, score | |
| <2 | 81 (60.4) |
| ≥2 | 53 (39.6) |
TNM, tumor-node-metastasis; SD, standard deviation; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PLT, platelet; FBG, fibrinogen; WBC, white blood cell; CO-NPF, combination of NLR, PLR, FBG and PLT.
The association between clinical characteristics and laboratory parameters with CO-NPF is presented in Table II. Significant differences between CO-NPF and age (P=0.010), TNM stage (P=0.034), neutrophil count (P=0.002), lymphocyte count (P=0.001), monocyte count (P=0.023), PLT count (P<0.001), FBG count (P<0.001), NLR (P<0.001) and PLR (P<0.001) were identified.
Table II.
Clinical characteristics and laboratory parameters according to CO-NPF.
| Characteristic | CO-NPF <2, n=81 (%) | CO-NPF ≥2, n=53 (%) | P-value |
|---|---|---|---|
| Age, years | 0.010 | ||
| <60 | 52 (70.3) | 22 (29.7) | |
| ≥60 | 29 (48.3) | 31 (51.7) | |
| Sex | 0.708 | ||
| Female | 31 (58.5) | 22 (41.5) | |
| Male | 50 (61.7) | 31 (38.3) | |
| Smoking index | 0.420 | ||
| <400 | 47 (63.5) | 27 (36.5) | |
| ≥400 | 34 (56.7) | 26 (43.3) | |
| Lymph node metastasis | 0.088 | ||
| Yes | 43 (54.4) | 36 (45.6) | |
| No | 38 (69.1) | 17 (30.9) | |
| TNM stage | 0.034 | ||
| I–II | 56 (67.5) | 27 (32.5) | |
| IIIA | 25 (49.0) | 26 (51.0) | |
| Operative approach | 0.777 | ||
| Local tumor resection | 7 (58.3) | 5 (41.7) | |
| Pulmonary lobectomy | 69 (61.6) | 43 (38.4) | |
| Pneumonectomy | 5 (50.0) | 5 (50.0) | |
| WBC count, 109 cells/l | 0.372 | ||
| <5.6 | 24 (66.7) | 12 (33.3) | |
| ≥5.6 | 57 (58.2) | 41 (41.8) | |
| Neutrophil count, 109 cells/l | 0.002 | ||
| <3.4 | 35 (79.5) | 9 (20.5) | |
| ≥3.4 | 46 (51.1) | 44 (48.9) | |
| Lymphocyte count, 109 cells/l | 0.001 | ||
| <2.30 | 52 (52.0) | 48 (48.0) | |
| ≥2.30 | 29 (85.3) | 5 (14.7) | |
| Monocyte count, 109 cells/l | 0.023 | ||
| <0.54 | 57 (67.9) | 27 (32.1) | |
| ≥0.54 | 24 (48.0) | 26 (52.0) | |
| PLT count, 109 cells/l | <0.001 | ||
| <289 | 74 (75.5) | 24 (24.5) | |
| ≥289 | 7 (19.4) | 29 (80.6) | |
| FBG count, g/l | <0.001 | ||
| <4 | 71 (79.8) | 18 (20.2) | |
| ≥4 | 10 (22.2) | 35 (77.8) | |
| NLR | <0.001 | ||
| <2.16 | 67 (90.5) | 7 (9.5) | |
| ≥2.16 | 14 (23.3) | 46 (76.7) | |
| PLR | <0.001 | ||
| <145 | 78 (86.7) | 12 (13.3) | |
| ≥145 | 3 (6.8) | 41 (93.2) |
TNM, tumor-node-metastasis; WBC, white blood cell; PLT, platelet; FBG, fibrinogen; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; CO-NPF, combination of NLR, PLR, FBG and PLT.
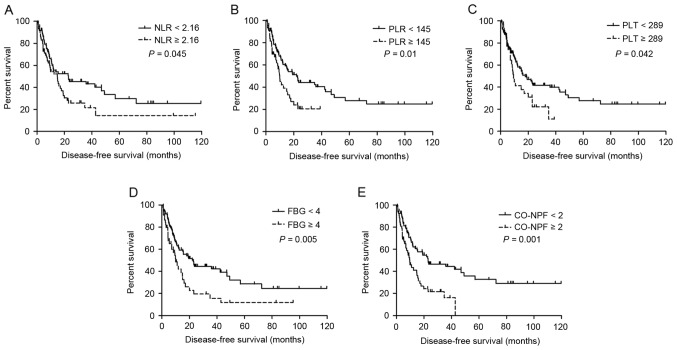
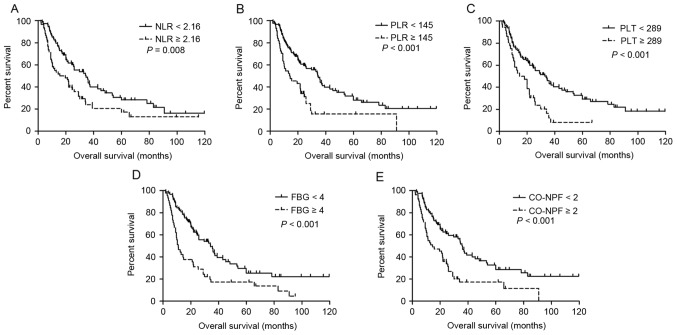
Univariate survival analysis results are summarized in Table III. Increased NLR (P=0.045 and P=0.008, respectively), PLR (P=0.010 and P<0.001, respectively), PLT (P=0.042 and P<0.001, respectively), FBG (P=0.005 and P<0.001, respectively) and CO-NPF (P=0.001 and P<0.001, respectively) were significantly associated with shorter DFS (Fig. 1A-E, respectively) and OS (Fig. 2A-E, respectively). Other significant prognostic factors for DFS and OS identified included lymph node metastasis, TNM stage, age and serum albumin (all P≤0.05).
Table III.
Univariate analysis of prognostic factors influencing OS and DFS.
| OS | DFS | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age, years (≥60 or <60) | 1.634 | 1.093–2.444 | 0.016 | 1.038 | 0.675–1.597 | 0.864 |
| Sex (male or female) | 0.935 | 0.620–1.411 | 0.749 | 0.854 | 0.552–1.321 | 0.477 |
| Smoking index (≥400 or <400) | 1.243 | 0.831–1.858 | 0.288 | 0.894 | 0.579–1.381 | 0.613 |
| Lymph node metastasis (yes or no) | 1.905 | 1.243–2.918 | 0.003 | 1.620 | 1.035–2.537 | 0.033 |
| TNM stage (I–II or IIIA) | 1.745 | 1.156–2.635 | 0.008 | 1.878 | 1.215–2.905 | 0.005 |
| Operative approach | 1.199 | 0.744–1.933 | 0.510 | 1.118 | 0.642–1.946 | 0.511 |
| Albumin (≥49.5 or <49.5 g/dl) | 2.078 | 1.076–4.010 | 0.026 | 1.978 | 0.952–4.110 | 0.062 |
| WBC count (≥5.6 or <5.6 ×109 cells/l) | 1.261 | 0.799–1.992 | 0.318 | 1.118 | 0.692–1.808 | 0.647 |
| NLR (≥2.16 or <2.16) | 1.724 | 1.150–2.584 | 0.008 | 1.551 | 1.005–2.395 | 0.045 |
| PLR (≥145 or <145) | 2.188 | 1.430–3.349 | <0.001 | 1.818 | 1.146–2.884 | 0.010 |
| PLT (≥289 or <289×109 cells/l) | 2.222 | 1.426–3.461 | <0.001 | 1.635 | 1.012–2.642 | 0.042 |
| FBG (≥4 or <4 g/l) | 2.131 | 1.417–3.206 | <0.001 | 1.875 | 1.202–2.925 | 0.005 |
| CO-NPF (≥2 or <2) | 2.187 | 1.451–3.295 | <0.001 | 2.156 | 1.380–3.370 | 0.001 |
Operative approaches were local tumor resection, pulmonary lobectomy or pneumonectomy. TNM, tumor-node-metastasis; HR, hazard ratio; CI, confidence interval; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PLT, platelet; FBG, fibrinogen; CO-NPF, combination of NLR, PLR, FBG and PLT; WBC, white blood cell; OS, overall survival; DFS, disease-free survival.
Figure 1.
Kaplan-Meier estimator curves of DFS for all patients with adenosquamous cancer. DFS rates according to (A) NLR, (B) PLR, (C) PLT, (D) FBG and (E) CO-NPF. DFS, disease-free survival; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PLT, platelet; FBG, fibrinogen; CO-NPF, combination of NLR, PLR, FBG and PLT.
Figure 2.
Kaplan-Meier estimator curves of OS for all patients with adenosquamous cancer. OS rates according to (A) NLR, (B) PLR, (C) PLT, (D) FBG and (E) CO-NPF. OS, overall survival; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PLT, platelet; FBG, fibrinogen; CO-NPF, combination of NLR, PLR, FBG and PLT.
Multivariate analysis results are presented in Table IV. CO-NPF and preoperative NLR, PLR, PLT and FBG were successively used for a multivariate Cox's regression model with other significant factors (excluding node stage) identified by univariate analyses. Increased CO-NPF (P=0.001 and P<0.001, respectively), PLR (P=0.011 and P=0.001, respectively) and FBG (P=0.001 and P<0.001, respectively) were identified to be independently and significantly associated with shorter DFS and OS. Other independent prognostic factors for OS were NLR (P=0.006), PLT (P=0.001), age (P=0.033) and serum albumin (P=0.003). TNM stage was identified to be independently associated with DFS (P<0.05), as presented in Table IV.
Table IV.
Multivariate analysis of prognostic factors influencing OS and DFS.
| OS | DFS | |||||
|---|---|---|---|---|---|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Model 1 | ||||||
| Age, years (≥60 or <60) | 1.586 | 1.039–2.423 | 0.033 | |||
| Albumin (≥49.5 or <49.5 g/dl) | 3.037 | 1.528–6.033 | 0.003 | |||
| TNM stage (I–II or IIIA) | 1.602 | 1.019–2.517 | 0.041 | |||
| CO-NPF (≥2 or <2) | 2.177 | 1.420–3.337 | <0.001 | 1.904 | 1.210–3.020 | 0.006 |
| Model 2 | ||||||
| Age, years (≥60 or <60) | 1.814 | 1.196–2.752 | 0.005 | |||
| Albumin (≥49.5 or <49.5 g/dl) | 2.731 | 1.357–5.494 | 0.005 | |||
| TNM stage (I–II or IIIA) | 1.621 | 1.069–2.460 | 0.023 | 1.878 | 1.215–2.905 | 0.005 |
| NLR (≥2.16 or <2.16) | 1.782 | 1.176–2.700 | 0.006 | |||
| Model 3 | ||||||
| Age, years (≥60 or <60) | 1.738 | 1.142–2.643 | 0.010 | |||
| Albumin (≥49.5 or <49.5 g/dl) | 2.615 | 1.313–5.211 | 0.006 | |||
| TNM stage (I–II or IIIA) | 1.538 | 1.006–2.351 | 0.047 | 1.715 | 1.097–2.681 | 0.018 |
| PLR (≥145 or <145) | 2.005 | 1.294–3.106 | 0.002 | 1.609 | 1.004–2.577 | 0.048 |
| Model 4 | ||||||
| Age, years (≥60 or <60) | 1.956 | 1.284–2.980 | 0.002 | |||
| Albumin (≥49.5 or <49.5 g/dl) | 2.099 | 1.046–4.214 | 0.037 | |||
| TNM stage (I–II or IIIA) | 1.878 | 1.215–2.905 | 0.005 | |||
| PLT (≥289 or <289 ×109 cells/l) | 2.276 | 1.432–3.617 | 0.001 | |||
| Model 5 | ||||||
| Age, years (≥60 or <60) | 1.553 | 1.009–2.389 | 0.045 | |||
| Albumin (≥49.5 or <49.5 g/dl) | 2.437 | 1.224–4.852 | 0.011 | |||
| TNM stage (I–II or IIIA) | 1.716 | 1.131–2.604 | 0.011 | 1.871 | 1.209–2.894 | 0.005 |
| FBG (≥4 or <4 g/l) | 2.045 | 1.332–3.141 | 0.001 | 1.865 | 1.196–2.910 | 0.006 |
TNM, tumor-node-metastasis; HR, hazard ratio; CI, confidence interval; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PLT, platelet; FBG, fibrinogen; CO-NPF, combination of NLR, PLR, FBG and PLT; OS, overall survival; DFS, disease-free survival.
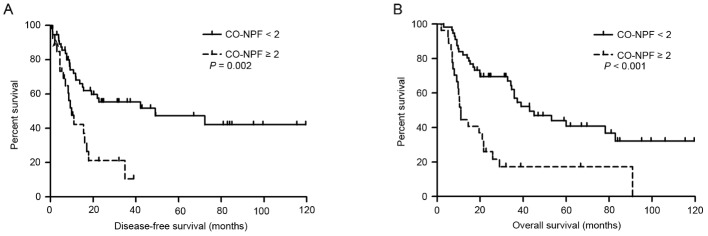
Subgroup analysis demonstrated that patients with CO-NPF <2 exhibited an improved prognosis compared with those with CO-NPF ≥2. Patients with CO-NPF <2 exhibited a 3-year OS rate of 46.9% and a 5-year OS rate of 28.6%, whereas those with CO-NPF ≥2 exhibited a 3-year OS rate of 17.4% and a 5-year OS rate of 11.6%. The median survival times of the two groups were 35.9 and 14.4 months, respectively (P<0.001; Fig. 2E). The prognostic impact of CO-NPF according to TNM stage (I–II or IIIA) was then analyzed and increased CO-NPF was identified to be significantly associated with shorter DFS and OS in the stage I–II subgroup (HR, 2.638; 95% CI, 1.422–4.894; P=0.002; and HR, 3.045; 95% CI, 1.742–5.324; P<0.001, respectively; Fig. 3).
Figure 3.
Kaplan-Meier estimator curves of DFS and OS rates of patients with stage I–II adenosquamous cancer according to CO-NPF. (A) DFS and (B) OS rates, according to CO-NPF of patients of TNM I–II stage. DFS, disease-free survival; OS, overall survival; CO-NPF, combination of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, fibrinogen and platelet; TNM, tumor-node-metastasis.
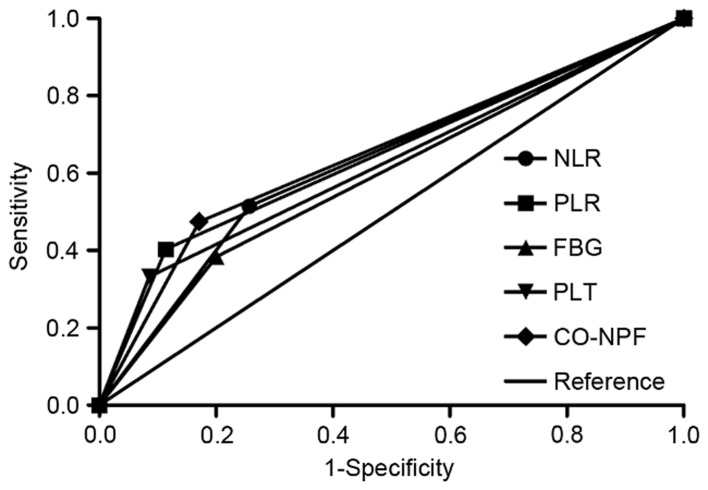
Finally, by comparing the AUC, the association between NLR, PLR, PLT, FBG and CO-NPF with 3-year OS rates was assessed (Fig. 4). CO-NPF exhibited greater predictive significance (AUC, 0.652; P=0.008; 95% CI, 0.551–0.752) compared with NLR (AUC, 0.629; P=0.024; 95% CI, 0.524–0.734), PLR (AUC, 0.645; P=0.011; 95% CI, 0.546–0.744), PLT (AUC, 0.624; P=0.030; 95% CI, 0.524–0.724) or FBG (AUC, 0.592; P=0.107; 95% CI, 0.486–0.697).
Figure 4.
Area under the curve of NLR, PLR, PLT, FBG and CO-NPF. Comparison of receiver operating characteristic curves based on NLR, PLR, PLT, FBG and CO-NPF in the 3-year overall survival rate. NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PLT, platelet; FBG, fibrinogen; CO-NPF, combination of NLR, PLR, FBG and PLT.
Discussion
The present retrospective study assessed the association between preoperative systemic inflammatory indexes and a novel inflammation-based marker (CO-NPF) with the postoperative prognosis of 134 patients with ASC. The results revealed that CO-NPF, NLR, PLR, PLT and FBG were independently and significantly associated with decreased OS rates. Furthermore, CO-NPF, PLR and FBG were all independent prognostic factors for DFS. CO-NPF was superior to NLR, PLR, PLT and FBG individually in predicting 3-year survival rates.
Systemic inflammatory responses may exert dual effects on cancer, promoting tumor proliferation, while inhibiting other oncogenic processes. The tumor microenvironment may be heavily infiltrated by inflammatory cells and increased pro-inflammatory cytokines released by these cells subsequently promote tumor growth (11). For example, neutrophils are able to significantly promote angiogenesis by secreting vascular endothelial growth factor (VEGF) and tumor progression by suppressing the cytotoxic activity of lymphocytes (11). Release of growth factors, including platelet factor 4, platelet-derived growth factor, thrombospondin and VEGF by platelets promotes hematogenous cancer spread, cancer cell adhesion, invasion, angiogenesis as well as tumor progression (25–27). In addition, fibrinogen may protect tumor cells from natural killer cytotoxicity (28). Conversely, lymphocytes suppress tumor growth and invasion through their cytolytic activity (29). Increased NLR may represent increased neutrophil counts, decreased lymphocyte counts, or both. The same is true for PLR with respect to the platelet and lymphocyte association. The prognostic significance of NLR, PLR, PLT and FBG has been suggested in numerous types of cancer including NSCLC. Although the prognostic role of these indexes in NSCLC remains controversial, the results of the present study are in line with a clinical prognostic value. Several studies have identified that increased pretreatment NLR or PLR predicts shorter progression-free survival (PFS), DFS and OS in patients with NSCLC as well as poor response to first-line platinum-based treatment (16,18,20). Furthermore, other reports suggest that increased pretreatment PLT and FBG are significantly associated with unfavorable DFS, PFS or OS in patients with NSCLC (13–15,19,21,22). Although the prognostic value of NLR, PLR, PLT and FBG in NSCLC is consistent with that in ASC, the exact threshold values of these indexes have not been defined. As a result, ROC curves were used in the present study to define the optimal threshold values.
NLR, PLR, PLT and FBG all represent systemic inflammation and serve important roles in cancer. CO-NPF may serve as a promising prognostic factor defining the association between inflammation and cancer in a more comprehensive manner. Indeed, CO-NPF was superior to NLR, PLR, PLT and FBG in predicting 3-year survival rates. Notably, subgroup analysis revealed that CO-NPF was significantly associated with decreased DFS and OS rates of patients in the stage I–II subgroup, but not of those in the stage IIIA subgroup (data not shown). This implies that the prognostic value of inflammatory indexes may be influenced by the TNM stage. By analyzing the association of clinical characteristics and laboratory parameters with CO-NPF, it was revealed that there were significant differences between the two CO-NPF groups in age, TNM stage, neutrophil count, lymphocyte count, monocyte count, PLT count, FBG count, NLR and PLR. However, CO-NPF was not significantly associated with lymph node metastasis, implying that inflammation is more likely to contribute to hematogenous metastasis, which is consistent with a previous study (30).
All patients recruited in the present study were from a single center which may limit the results, thus these results require further confirmation by a multi-center study. In addition, as a retrospective study, certain useful parameters which were not routinely measured prior to surgery may have been missed. For example, C-reactive protein measurement (30) and Glasgow Prognostic Score (31) may be used to confirm the results in a prospective study. Wu et al (32) and Kwon et al (33), respectively, demonstrated that phosphorylated insulin-like growth factor-1 receptor (pIGF1R) and mucin 4 expression were associated with the prognosis of patients with pulmonary adenocarcinoma. However, the optimum prognostic factor among CO-NPF, mucin 4 and plGF1R requires further confirmation by future studies. However, to the best of our knowledge, the present study is the first to investigate the prognostic role of PLT, FBG, NLR and PLR in patients with ASC, and the results of the present study further demonstrated that CO-NPF has improved predictive power compared with NLR, PLR, PLT and FBG individually. These inflammatory indexes are cost-effective, readily available and effective prognostic factors, with the results of the present study suggesting that assessing them together is superior to their use independently.
Acknowledgements
The authors are grateful to Dr Douglas E. Linn (Brigham & Women's Hospital, Boston, MA, USA) for a critical reading of the paper. The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81372517 and 81000899), the Tianjin Municipal Science and Technology Commission Key Application Research Projects (grant no. 11JCZDJC18900), the National Natural Science Foundation of China (grant no. 81501983), the National Science and Technology Major Project (grant no. 2013ZX) and the Tianjin Municipal Science and Technology Commission Projects (grant nos. 11JCYBJC11300 and 12ZCDZSY15600).
References
- 1.Chang S, Dai M, Ren JS, Chen YH, Guo LW. Estimates and prediction on incidence, mortality and prevalence of lung cancer in China in 2008. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33:391–394. (In Chinese) [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. In J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgibbons PL, Kern WH. Adenosquamous carcinoma of the lung: A clinical and pathologic study of seven cases. Hum Pathol. 1985;16:463–466. doi: 10.1016/S0046-8177(85)80083-6. [DOI] [PubMed] [Google Scholar]
- 4.Ishida T, Kaneko S, Yokoyama H, Inoue T, Sugio K, Sugimachi K. Adenosquamous carcinoma of the lung. Clinicopathologic and immunohistochemical features. Am J Clin Pathol. 1992;97:678–685. doi: 10.1093/ajcp/97.5.678. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu J, Oda M, Hayashi Y, Nonomura A, Watanabe Y. A clinicopathologic study of resected cases of adenosquamous carcinoma of the lung. Chest. 1996;109:989–994. doi: 10.1378/chest.109.4.989. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs AR, Thunnissen FB. Histological typing of lung and pleural tumours: Third edition. J Clin Pathol. 2001;54:498–499. doi: 10.1136/jcp.54.7.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sridhar KS, Bounassi MJ, Raub W, Jr, Richman SP. Clinical features of adenosquamous lung carcinoma in 127 patients. Am Rev Respir Dis. 1990;142:19–23. doi: 10.1164/ajrccm/142.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y, Chung JH, Kim SE, Kim TJ, Lee KW. Adenosquamous carcinoma of the lung: CT, FDG PET, and clinicopathologic findings. Clin Nucl Med. 2014;39:107–112. doi: 10.1097/RLU.0b013e3182952c2d. [DOI] [PubMed] [Google Scholar]
- 9.Maeda H, Matsumura A, Kawabata T, Suito T, Kawashima O, Watanabe T, Okabayashi K, Kubota I. Japan National Hospital Organization Study Group for Lung Cancer: Adenosquamous carcinoma of the lung: Surgical results as compared with squamous cell and adenocarcinoma cases. Eur J Cardiothorac Surg. 2012;41:357–361. doi: 10.1016/j.ejcts.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 10.Mordant P, Grand B, Cazes A, Foucault C, Dujon A, Le Pimpec Barthes F, Riquet M. Adenosquamous carcinoma of the lung: Surgical management, pathologic characteristics, and prognostic implications. Ann Thorac Surg. 2013;95:1189–1195. doi: 10.1016/j.athoracsur.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 13.Ji Y, Sheng L, Du X, Qiu G, Su D. Elevated platelet count is a strong predictor of poor prognosis in stage I non-small cell lung cancer patients. Platelets. 2015;26:138–142. doi: 10.3109/09537104.2014.888547. [DOI] [PubMed] [Google Scholar]
- 14.Jiang HG, Li J, Shi SB, Chen P, Ge LP, Jiang Q, Tang XP. Value of fibrinogen and D-dimer in predicting recurrence and metastasis after radical surgery for non-small cell lung cancer. Med Oncol. 2014;31:22. doi: 10.1007/s12032-014-0022-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim KH, Park TY, Lee JY, Lee SM, Yim JJ, Yoo CG, Kim YW, Han SK, Yang SC. Prognostic significance of initial platelet counts and fibrinogen level in advanced non-small cell lung cancer. J Korean Med Sci. 2014;29:507–511. doi: 10.3346/jkms.2014.29.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kos M, Hocazade C, Kos FT, Uncu D, Karakas E, Dogan M, Uncu HG, Yildirim N, Zengin N. Prognostic role of pretreatment platelet/lymphocyte ratio in patients with non-small cell lung cancer. Wien Klin Wochenschr. 2016;128:635–640. doi: 10.1007/s00508-015-0724-8. [DOI] [PubMed] [Google Scholar]
- 17.Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ, Lee JH. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17:216–222. doi: 10.3109/1354750X.2012.656705. [DOI] [PubMed] [Google Scholar]
- 18.Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110:1930–1935. doi: 10.1038/bjc.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheng L, Luo M, Sun X, Lin N, Mao W, Su D. Serum fibrinogen is an independent prognostic factor in operable nonsmall cell lung cancer. Int J Cancer. 2013;133:2720–2725. doi: 10.1002/ijc.28284. [DOI] [PubMed] [Google Scholar]
- 20.Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471–479. doi: 10.1007/s00262-012-1347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu D, Liu B, Zhang L, Du K. Platelet count predicts prognosis in operable non-small cell lung cancer. Exp Ther Med. 2013;5:1351–1354. doi: 10.3892/etm.2013.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu JF, Cai L, Zhang XW, Wen YS, Su XD, Rong TH, Zhang LJ. High plasma fibrinogen concentration and platelet count unfavorably impact survival in non-small cell lung cancer patients with brain metastases. Chin J Cancer. 2014;33:96–104. doi: 10.5732/cjc.012.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev. 2013;14:5237–5242. doi: 10.7314/APJCP.2013.14.6.3945. [DOI] [PubMed] [Google Scholar]
- 24.Vallières E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z, Goldstraw P. International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions: The IASLC Lung Cancer Staging Project: Proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:1049–1059. doi: 10.1097/JTO.0b013e3181b27799. [DOI] [PubMed] [Google Scholar]
- 25.Dubernard V, Arbeille BB, Lemesle MB, Legrand C. Evidence for an alpha-granular pool of the cytoskeletal protein alpha-actinin in human platelets that redistributes with the adhesive glycoprotein thrombospondin-1 during the exocytotic process. Arterioscler Thromb Vasc Biol. 1997;17:2293–2305. doi: 10.1161/01.ATV.17.10.2293. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins: Studies on release and subcellular localization. Blood. 1979;53:604–618. [PubMed] [Google Scholar]
- 27.Qian X, Tuszynski GP. Expression of thrombospondin-1 in cancer: A role in tumor progression. Proc Soc Exp Biol Med. 1996;212:199–207. doi: 10.3181/00379727-212-44008. [DOI] [PubMed] [Google Scholar]
- 28.Zheng S, Shen J, Jiao Y, Liu Y, Zhang C, Wei M, Hao S, Zeng X. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100:859–865. doi: 10.1111/j.1349-7006.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Huang SH, Li H, Li Y, Chen XL, Zhang WQ, Chen HG, Gu LJ. Preoperative lymphocyte count is a favorable prognostic factor of disease-free survival in non-small-cell lung cancer. Med Oncol. 2013;30:352. doi: 10.1007/s12032-012-0352-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Zhang L, Zhu K, Shi B, Yin Y, Zhu J, Yue D, Zhang B, Wang C. Prognostic significance of combination of preoperative platelet count and neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small cell lung cancer: Based on a large cohort study. PLoS One. 2015;10:e0126496. doi: 10.1371/journal.pone.0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillan DC. The systemic inflammation-based Glasgow prognostic score: A decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Wu PF, Huang WC, Yang JC, Lu YS, Shih JY, Wu SG, Lin CH, Cheng AL. Phosphorylated insulin-like growth factor-1 receptor (pIGF1R) is a poor prognostic factor in brain metastases from lung adenocarcinomas. J Neurooncol. 2013;115:61–70. doi: 10.1007/s11060-013-1194-3. [DOI] [PubMed] [Google Scholar]
- 33.Kwon KY, Ro JY, Singhal N, Killen DE, Sienko A, Allen TC, Zander DS, Barrios R, Haque A, Cagle PT. MUC4 expression in non-small cell lung carcinomas: Relationship to tumor histology and patient survival. Arch Pathol Lab Med. 2007;131:593–598. doi: 10.5858/2007-131-593-MEINCL. [DOI] [PubMed] [Google Scholar]