Abstract
Cholesterol increases the risk of colorectal cancer. Liver X receptor (LXR), retinoid X receptor (RXR)α and sterol regulatory element binding protein (SREBP)-1c are transcriptional regulators of lipid metabolism. Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) serves an essential role in angiogenesis and development, but its role in cancer is controversial. The expression of COUP-TFII, LXR, RXRα and SREBP-1c in colorectal cancer, as well as their association with clinicopathologic features, was assessed, and their utility as prognostic indicators in colorectal cancer evaluated. Colorectal cancer samples (n=707 patients) were analyzed for COUP-TII, LXR, RXRα and SREBP-1c expression by immunohistochemistry. Overall survival curves of patients with tumors expressing different levels of these proteins were produced and risk factors were assessed. Of the 707 patients, 32.7, 50.9, 56.4, and 41.7% were positive for COUP-TFII, LXR, RXRα, and SREBP-1c, respectively. The lack of COUP-TFII or LXR expression was associated with lower overall survival rates (P=0.0154 for COUP-TFII, and 0.0113 for LXR). Following adjustment for other clinical risk factors (age, sex, tumor size, grade, vascular invasion, and Tumor-Node-Metastasis stage), the lack of COUP-TFII or LXR expression was a negative independent prognostic factor for survival. The expression of COUP-TFII and LXR alone or in combination may be biomarkers to indicate a positive prognosis in patients with colorectal cancer.
Keywords: colorectal cancer, chicken ovalbumin upstream promoter-transcription factor II, liver X receptor, retinoid X receptor α, prognosis
Introduction
Colorectal cancer is one of the most common types of cancer worldwide (1). Despite advances in diagnostic and therapeutic strategies, clinical outcomes and prognoses for patients with colorectal cancer remain unsatisfactory (2). Therefore, the identification of molecular markers for the more aggressive colorectal tumor phenotypes is required, to allow patient treatment to be adjusted accordingly. However, predictive molecular indicators of regional disease invasion and metastasis are not well defined.
Nuclear receptors (NRs) are ligand-activated transcription factors that control the expression of genes involved in nearly all aspects of development, physiology, and disease (3). The majority of NRs are receptors for small lipophilic ligands (metabolites, hormones, drugs, and environmental compounds) that directly modulate their transcriptional activities (4). The NRs liver X receptor α (LXRα) and β are key regulators of lipid, cholesterol and carbohydrate metabolism and homeostasis (5). They function as transcription factors by heterodimerizing with retinoid X receptor (RXR) and increasing the expression of target genes that encode proteins implicated in lipid metabolism, particularly in cholesterol efflux and fatty acid synthesis (6). Cholesterol controls cell proliferation; disruptions in cholesterol metabolism are associated with the development of colon cancer (7). Previous studies have indicated that LXRs may couple cholesterol homeostasis to proliferation (8–14). Synthetic (compounds T0901317 and GW3965) and natural (22[R]-hydroxycholesterol and 24[S]-hydroxycholesterol) LXR ligands suppress the proliferation of a number of human cancer cell lines, including prostate, breast, colon, ovarian and leukemia cancer cells (8–14). Furthermore, downregulation of the S-phase-associated kinase protein-2 (Skp2) component of ubiquitin ligase, which regulates p27Kip1 degradation (15) and the resulting p27Kip1 protein stabilization and retinoblastoma protein dephosphorylation, may contribute to the inhibition of cell proliferation (16). In addition, LXRs inhibit the proliferation of human colorectal cancer cells and the growth of intestinal tumors in mice (7).
RXR is an NR family member that has been implicated in cancer chemoprevention (17,18). RXRα expression is decreased in mouse skin tumors (19), whereas RXRβ expression is increased in non-small cell lung tumors (20) compared with healthy tissue. However, the clinical significance of RXR in colorectal cancer remains unclear.
Sterol regulatory element-binding protein-1c (SREBP-1c) is a transcriptional intermediary for the insulin stimulation of fatty acid synthase (FAS) gene expression (21). Induction of FAS expression and the consequential enhanced fatty acid synthesis is required for neoplastic transformation and tumor progression (22). SREBP-1 may be implicated in tumorigenesis, as the high expression of SREBP-1 is reported to predict a poor prognosis in patients with pancreatic cancer (23).
The orphan NR chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) is involved in the regulation of gene expression (24,25), development, differentiation, and homeostasis (26); however, its role in cancer is debated as contradictory tumor-suppressive and oncogenic capacities have been reported (27,28). Increased expression of COUP-TFII was shown to enhance the invasiveness of human lung carcinoma cells (27). By contrast, a previous report demonstrated that overexpression of COUP-TFII in MDA-MB-435 breast cancer cells led to reduced growth and plating efficiency (28). The prognostic significance of high or low expression of COUP-TFII appears to vary; its expression may be a favorable (e.g., ovarian and colon cancer) or an unfavorable (e.g., breast and prostate cancer) prognostic factor in patients with different types of cancer, and its expression is tumor-specific (29). The underlying mechanisms that trigger altered expression of this gene in individual tumors remains poorly understood (30–32). According to a previous study, patients with COUP-TFII-positive tumors had a significantly higher 3-year overall survival (OS) rate compared with the COUP-TFII-negative group (33). However, the follow-up period was short and few patients with colorectal cancer were included in the study. Therefore, in the present study, the aim was to investigate the association between COUP-TFII expression and clinicopathological factors further and to confirm its prognostic significance in a larger number of patients with colorectal cancer. The association between LXR, RXRα, and SREBP-1c expression and clinicopathological factors was also assessed in the study participants.
Materials and methods
Patients and tissue samples
Consecutive patients with colorectal cancer who were eligible and underwent surgery at Dong-A University Hospital between March 2002 and July 2011 (n=707) were enrolled in the study, including 403 males (age range, 29.0–87.0 years; mean age, 61.8 years) and 304 females (age range, 22.0–84.0 years; mean age, 62.1 years). Tissue samples from the patients were formalin-fixed and paraffin-embedded. Patients with familial adenomatous polyposis or inflammatory bowel disease or synchronous colorectal or extracolorectal cancer, and those lost to follow-up, were excluded. None of the patients had a family history of colorectal cancer, and none had received preoperative chemotherapy or radiotherapy. Information concerning age, sex, histological grade and Tumor-Node-Metastasis (TNM) stage (34) was retrieved by reviewing pathological and surgical reports. The present study was approved by the institutional review board of Dong-A University (Busan, Korea; approval no., 2-104709-AB-N-01-201504-BR-004-02).
Tissue microarrays and immunohistochemistry
Cores (1 mm) were removed from colorectal cancer samples that had previously been formalin-fixed and paraffin-embedded. For all arrays, three cores from different areas of the tumor were collected and placed in a new blank recipient paraffin block, according to a previously described method (35). Sections were deparaffinized using a series of xylene baths; rehydration was performed using a series of graded alcohol solutions. Sections (4-µm thick) were used for immunohistochemical staining. To enhance immunoreactivity, microwave antigen retrieval was performed at 750 W for 30 min in Tris EDTA (pH 9.0). Subsequent to blocking endogenous peroxidase activity with 5% hydrogen peroxidase for 10 min, incubation with the primary antibody was performed for 1 h at room temperature. The primary antibodies used in immunostaining included a mouse monoclonal antibody directed against COUP-TFII (clone H7147; catalog no., PP-H7147-00; 1:100; Perseus Proteomics Inc., Tokyo, Japan), a rabbit polyclonal antibody directed against LXRα/β (clone S-20; catalog no., sc-1000; 1:400; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), a mouse monoclonal antibody directed against RXRα (clone F-1; catalog no., sc-46659; 1:50; Santa Cruz Biotechnology, Inc.), and a rabbit polyclonal antibody directed against SREBP-1 (clone H-160; catalog no., sc-8984; 1:100; Santa Cruz Biotechnology, Inc.). An Envision™Chem™ Detection kit (DakoCytomation, Carpinteria, CA, USA) was used for the secondary antibody at room temperature for 30 min. After washing the tissue samples in TBS for 10 min, 3,3′-diaminobenzidine was used as a chromogen, and then Mayer's hematoxylin counterstain was applied for 1 min at room temperature. Archival, 10% formalin-fixed (for 18–48 h at room temperature), paraffin-embedded human normal kidney, thyroid, skin and testis tissues (obtained from tissue archives at Dong-A University Hospital) were used as positive controls for COUP-TFII, LXRα/β, RXRα, and SREBP-1c, according to the antibody manufacturer's protocol. A negative control was obtained by substituting the primary antibody with buffer.
Immunohistochemical assessment
The percentage and intensity of immunoreactive tumor cells in each core were recorded, and the final value of the positive tumor cells was determined as the mean of the immunoreactivity of the three cores. The presence of tumor tissue in ≥2 interpretable cores was required for the inclusion of a case in statistical analyses. All slides were independently evaluated by two independent experienced pathologists (MSR and MGP) who were blinded to clinicopathological data. There were only minor discrepancies in the evaluation; slides with discrepancies between evaluations were reevaluated under a multi-head microscope until a consensus evaluation was obtained. The percentage of positive tumor cells and the staining intensity (weak or strong) were assessed. Staining intensity was scored visually and stratified as follows: Negative, weak (if the staining appeared as a blush), or strong (if it was markedly positive at 20x magnification).
For COUP-TFII, immunoreactivity was defined as cells showing nuclear staining in the tumor tissue with minimal background staining. Tumors with strong staining intensity in >10% of tumor cells were recorded as having positive immunoreactivity for COUP-TFII. For LXRα/β, immunoreactivity was defined as cells exhibiting nuclear staining with/without cytoplasmic staining patterns in the tumor tissue with minimal background staining; tumors with strong staining intensity in >10% of the tumor cells were recorded as having positive immunoreactivity for LXRα/β. For RXRα, immunoreactivity was defined as cells exhibiting nuclear staining in the tumor tissue with minimal background staining. Cases were divided into those with weak or strong RXRα expression according to staining intensity, since immunoreactivity was typically evenly distributed within a tumor sample, but varied in intensity. For SREBP-1c, immunoreactivity was defined as cells exhibiting nuclear staining with/without cytoplasmic staining patterns in the tumor tissue with minimal background staining; tumors with a strong staining intensity in >10% of tumor cells were recorded as having positive immunoreactivity for SREBP-1c.
Statistical analysis
The χ2 test was used to analyze differences in clinical characteristics and immunohistochemically-assessed expression levels. Survival curves were calculated by the Kaplan-Meier method, and comparisons of survival curves were made with the log-rank test. Multiple analyses were performed with the Cox proportional hazards model to assess the association of COUP-TFII, LXR, RXRα, and SREBP-1c expression with the OS rate. P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed with SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
Expression of COUP-TFII, LXR, RXRα, and SREBP-1c in human colorectal cancer tissues
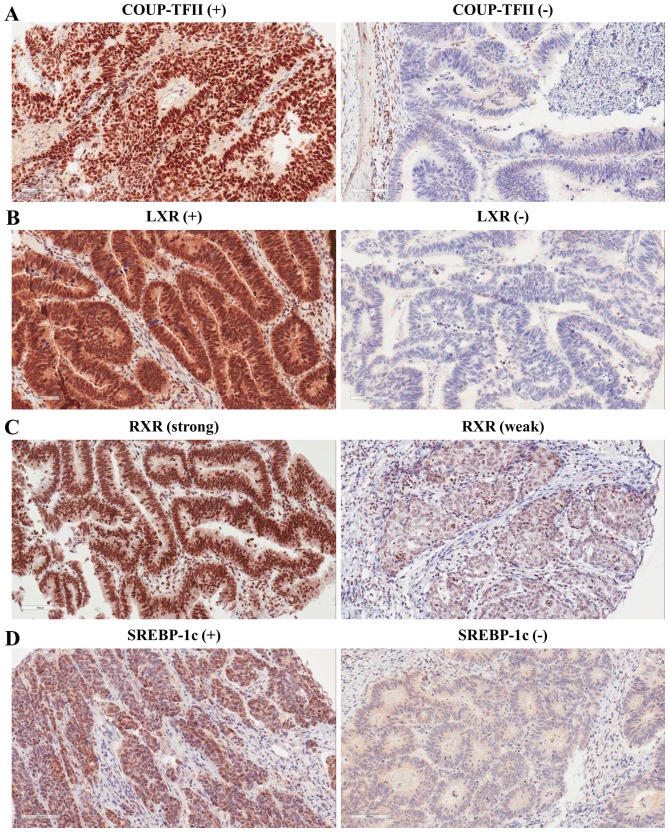
Our previous study revealed expression of COUP-TFII in 55/95 (57.89%) colorectal carcinoma tissue specimens (33). To confirm the expression pattern of COUP-TFII in a larger number of patients with human colorectal carcinoma, immunohistochemistry was performed with an antibody against COUP-TFII. Positive COUP-TFII expression was observed in 231/707 (32.7%) colorectal carcinoma tissue specimens. Immunostaining occurred predominantly in the nuclei of tumor cells (Fig. 1). The expression levels of LXR, RXRα, and SREBP-1c were also assessed in human colorectal tumors by immunohistochemistry. Positive expression of LXR and SREBP-1c was observed in 360 (50.9%) and 295 (41.7%) of the 707 colorectal carcinoma tissue specimens, respectively (Fig. 1; Table I). Positive expression of RXRα was observed in 399/704 (56.4%) colorectal carcinoma tissue specimens (Fig. 1 and Table I). Core tissue was lost during the preparation of three colorectal carcinoma tissue specimens.
Figure 1.
Representative images of immunohistochemical staining for COUP-TFII, LXR, RXRα, and SREBP-1c in colorectal cancer tissue. (A) Left, COUP-TFII-positive colorectal cancer tissue. Right, COUP-TFII-negative colorectal cancer tissue. (B) Left, LXR-positive colorectal cancer tissue. Right, LXR-negative colorectal cancer tissue. (C) Left, strong RXRα immunoreactivity detected in well-differentiated colorectal cancer tissues. Right, weak RXRα immunoreactivity detected in poorly differentiated colorectal cancer tissues. (D) Left, SREBP-1c-positive colorectal cancer tissue. Right, SREBP-1c-negative colorectal cancer tissue. Magnification, ×200. COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; LXR, liver X receptor; RXRα, retinoid X receptor α; SREBP-1c, sterol regulatory element binding protein-1c.
Table I.
Clinical characteristics and immunohistochemistry expressions of the study participants (n=707).
| Variable | Patients, n (%) |
|---|---|
| Sex | |
| Male | 403 (57.0) |
| Female | 304 (43.0) |
| Age, years | |
| <65 | 392 (55.5) |
| ≥65 | 315 (44.6) |
| Grade | |
| 1 | 398 (56.3) |
| 2 | 262 (37.1) |
| 3+4 | 47 (6.7) |
| Tumor size, cm | |
| <5 | 247 (34.9) |
| ≥5 | 460 (65.1) |
| Vascular invasion | |
| Negative | 605 (85.6) |
| Positive | 102 (14.4) |
| TNM stage | |
| 0+I | 95 (13.4) |
| II | 295 (41.7) |
| III+IV | 317 (44.8) |
| COUP-TEII expression | |
| Negative | 476 (67.3) |
| Positive | 231 (32.7) |
| LXR expression | |
| Negative | 347 (49.1) |
| Positive | 360 (50.9) |
| RXRα expression | |
| No dataa | 3 (0.4) |
| Negative | 305 (43.1) |
| Positive | 399 (56.4) |
| SREBP-1c expression | |
| Negative | 412 (58.3) |
| Positive | 295 (41.7) |
Three samples are missing in the RXRα immunohistochemical data. COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; LXR, liver X receptor; RXRα, retinoid X receptor α; SREBP-1c, sterol regulatory element binding protein-1c.
To evaluate the associations between COUP-TFII expression and LXR, RXRα, and SREBP-1c expression in colorectal cancer, the χ2 test was used. In 70.7% (163/231) of patient samples in which COUP-TFII was expressed, LXR was also expressed (P<0.0001). In 64.1% (148/229) of patient samples in which COUP-TFII was expressed, RXRα was also expressed (P=0.0035). In 49.8% (115/231) of patient samples in which COUP-TFII was expressed, SREBP-1c was also expressed (P=0.0027; Table II). These data suggest that COUP-TFII expression is positively associated with LXR, RXRα and SREBP-1c expression.
Table II.
Differential distribution of LXR, RXRα, and SREBP-1c according to COUP-TFII expression.
| LXR expression, n (%) | RXRα expressionb, n (%) | SREBP-1c expression, n (%) | ||||
|---|---|---|---|---|---|---|
| COUP-TFII expression | Negative | Positive | Negative | Positive | Negative | Positive |
| Negative | 279 (58.6) | 197 (41.4) | 224 (47.1) | 251 (52.7) | 296 (62.2) | 180 (37.8) |
| Positive | 68 (29.4) | 163 (70.7) | 81 (35.1) | 148 (64.1) | 116 (50.2) | 115 (49.8) |
| P-valuea | <0.0001 | 0.0035 | 0.0027 | |||
Calculated by χ2 test.
Three samples are missing in the RXRα immunohistochemical data. COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; LXR, liver X receptor; RXRα, retinoid X receptor α; SREBP-1c, sterol regulatory element binding protein-1c.
Associations between the expression of COUP-TFII, LXR, RXRα and SREBP-1c, and clinicopathological features
Following the analysis of COUP-TFII, LXR, RXRα and SREBP-1c staining in tumors, the χ2 test was used to evaluate the association between COUP-TFII, LXR, RXRα and SREBP-1c expression, and the clinicopathological features of the study population. As shown in Table III, there was a significant association of vascular invasion (P=0.0184) and the TNM stage (P=0.0215) with COUP-TFII expression, and samples that exhibited vascular invasion and higher TNM stages tended to be COUP-TFII-negative. No significant association was identified between COUP-TFII expression and the patient's age or sex, or the tumor size or grade (Table III). There was a significant negative association between LXR expression and vascular invasion (P=0.0334). No significant association was found between LXR expression and the patient's age or sex, the tumor size or grade, or the TNM stage (Table III). There was a significant association between tumor grade (P=0.0160) and RXRα expression, with high grades tending to be RXRα-negative. No significant association was identified between RXRα expression and age, sex, TNM stage, tumor size or vascular invasion (Table III). No significant association was identified between SREBP-1c expression and age at the time of surgery, sex, size, grade, TNM stage or vascular invasion (Table III).
Table III.
Univariate analysis of the associations between clinical characteristics and COUP-TFII, LXR, RXRα, and SREBP-1c expression.
| COUP-TFII | LXR | RXRα | SREBP-1c | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Positive, n (%) | P-value | Positive, n (%) | P-value | Positive, n (%) | P-value | Positive, n (%) | P-value |
| Sex | 0.3883 | 0.7376 | 0.7935 | 0.4272 | ||||
| Male | 137 (34.0) | 203 (50.4) | 225 (56.3) | 163 (40.5) | ||||
| Female | 94 (30.9) | 157 (51.6) | 174 (57.2) | 132 (43.4) | ||||
| Age, years | 0.8618 | 0.0790 | 0.8739 | 0.4837 | ||||
| <65 | 127 (32.4) | 188 (48.0) | 220 (56.4) | 159 (40.6) | ||||
| ≥65 | 104 (33.0) | 172 (54.6) | 179 (57.0) | 136 (43.2) | ||||
| Grade | 0.2914 | 0.2749 | 0.0160 | 0.7015 | ||||
| 1 | 134 (33.7) | 196 (49.3) | 240 (60.5) | 171 (43.0) | ||||
| 2 | 78 (29.8) | 143 (54.6) | 141 (53.8) | 104 (39.7) | ||||
| 3+4 | 19 (40.4) | 21 (44.7) | 18 (40.0) | 20 (42.6) | ||||
| Tumor size, cm | 0.6494 | 0.6620 | 0.2653 | 0.0537 | ||||
| <5 | 153 (33.3) | 237 (51.5) | 266 (58.2) | 204 (44.4) | ||||
| ≥5 | 78 (31.6) | 123 (49.8) | 133 (53.9) | 91 (36.8) | ||||
| Vascular invasion | 0.0184 | 0.0334 | 0.6958 | 0.7546 | ||||
| Negative | 208 (34.4%) | 42 (41.2) | 343 (57.0) | 44 (43.1) | ||||
| Positive | 23 (22.6%) | 318 (52.6) | 56 (54.9) | 251 (41.5) | ||||
| TNM stage | 0.0215 | 0.3616 | 0.5857 | 0.6859 | ||||
| 0+I | 38 (40.0) | 51 (53.7) | 51 (53.7) | 36 (37.9) | ||||
| II | 106 (35.9) | 157 (53.2) | 173 (58.8) | 123 (41.7) | ||||
| III+IV | 87 (27.4) | 152 (48.0) | 175 (55.6) | 136 (42.9) | ||||
COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; LXR, liver X receptor; RXRα, retinoid X receptor α; SREBP-1c, sterol regulatory element binding protein-1c.
Association of COUP-TFII and LXR expression with good prognosis in colorectal cancer patients
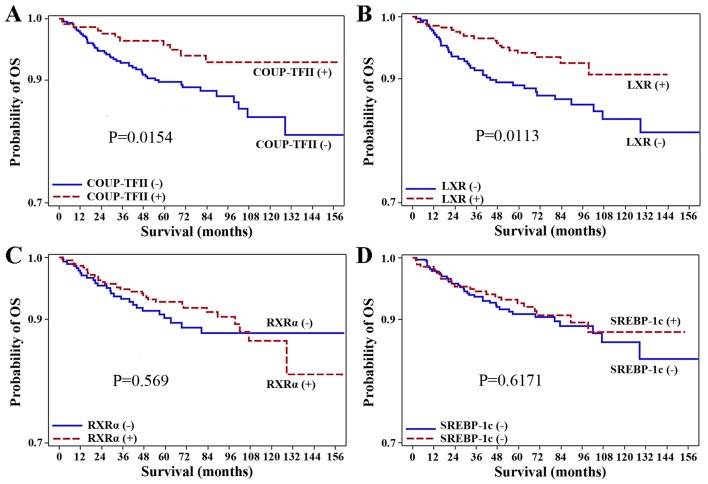
To assess whether COUP-TFII expression is a significant prognostic factor for the survival of patients with surgically resected colorectal carcinoma, a log-rank test was used with Kaplan-Meier survival curves. The median follow-up duration was 63.39 months. Of the 707 patients analyzed, the patients positive for COUP-TFII expression (231 patients) had a significantly higher OS rate than those negative for COUP-TFII expression (P=0.0154; Fig. 2). Similarly, the positive expression of LXR was associated with better OS rate (P=0.0113; Fig. 2). However, the positive expression of RXRα and SREBP-1c were not associated with the OS rate (P=0.569, P=0.6171, respectively). Additionally, Cox proportional hazards regression analysis revealed that the negative expression of LXR [hazard ratio (HR), 1.99; 95% confidence interval (CI), 1.16–3.42; P=0.0130] or COUP-TFII (HR, 2.20; 95% CI, 1.14–4.24; P=0.0182) were associated with significantly worse prognoses than the positive expression of LXR or COUP-TFII (Tables IV and V).
Figure 2.
Kaplan-Meier OS curves for 707 patients with colorectal cancer, according to expression levels of COUP-TFII, LXR, RXRα, and SREBP-1c. (A) Patients stratified according to COUP-TFII expression. (B) Patients stratified according to LXR expression. (C) Patients stratified according to RXRα expression. (D) Patients stratified according to SREBP-1c expression. OS rates are indicated in each panel. OS, overall survival; COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; LXR, liver X receptor; RXRα, retinoid X receptor α; SREBP-1c, sterol regulatory element binding protein-1c.
Table IV.
Crude HRs for COUP-TFII, LXR, RXRα, and SREBP-1c expression.
| Expression status | HR | 95% CI | P-value | |
|---|---|---|---|---|
| COUP-TFII | ||||
| Negative | 2.20 | 1.14, 4.24 | 0.0182 | |
| Positive | ref. | |||
| LXR | ||||
| Negative | 1.99 | 1.16, 3.42 | 0.0130 | |
| Positive | ref. | |||
| RXRα | ||||
| Negative | 1.16 | 0.69, 1.95 | 0.5693 | |
| Positive | ref. | |||
| SREBP-1c | ||||
| Negative | 1.14 | 0.68, 1.93 | 0.6174 | |
| Positive | ref. | |||
| COUP-TFII + LXR | ||||
| C(−)L(−) | 2.43 | 1.17, 5.04 | 0.0171 | |
| C(−)L(+) | 1.05 | 0.43, 2.53 | 0.9201 | |
| C(+)L(−) | 0.50 | 0.11, 2.33 | 0.3800 | |
| C(+)L(+) | ref. |
COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; LXR, liver X receptor; RXRα, retinoid X receptor α; SREBP-1c, sterol regulatory element binding protein-1c; HR, hazard ratio; CI: confidence interval; ref., hazard ratio reference value of 1; C, COUP-TFII; R, RXRα; L, LXR.
Table V.
HRs for COUP-TFII, LXR, or RXRα expression and clinical characteristics.
| Multiple models | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude model | COUP-TFII | LXR | RXRα | SREBP-1c | |||||||||||
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Expression | |||||||||||||||
| Negative | 2.16 | 1.11–4.23 | 0.0243 | 1.83 | 1.06–3.17 | 0.0306 | 1.16 | 0.69–1.95 | 0.5822 | 1.20 | 0.71–2.03 | 0.5076 | |||
| Sex | |||||||||||||||
| Male | 1.80 | 1.03–3.13 | 0.0381 | 1.88 | 1.08–3.29 | 0.0266 | 1.80 | 1.03–3.13 | 0.0399 | 1.76 | 1.00–3.07 | 0.0490 | 1.81 | 1.03–3.15 | 0.0378 |
| Age, years | |||||||||||||||
| <65 | 1.48 | 0.85–2.58 | 0.1641 | 1.39 | 0.80–2.43 | 0.2425 | 1.33 | 0.76–2.33 | 0.3130 | 1.37 | 0.78–2.40 | 0.2678 | 1.39 | 0.80–2.43 | 0.2441 |
| Grade | |||||||||||||||
| 3+4 | 1.69 | 0.66–4.44 | 0.2746 | 1.89 | 0.72–4.95 | 0.1954 | 1.57 | 0.61–4.07 | 0.3521 | 1.30 | 0.46–3.73 | 0.6213 | 1.56 | 0.60–4.03 | 0.3601 |
| 2 | 1.19 | 0.69–2.04 | 0.5370 | 0.95 | 0.55–1.65 | 0.8519 | 0.98 | 0.56–1.71 | 0.9370 | 0.92 | 0.53–1.62 | 0.7826 | 0.93 | 0.54–1.62 | 0.8014 |
| Tumor size | |||||||||||||||
| ≥5 cm | 1.30 | 0.75–2.26 | 0.3591 | 1.19 | 0.67–2.12 | 0.5459 | 1.19 | 0.67–2.11 | 0.5454 | 1.16 | 0.66–2.07 | 0.6057 | 1.19 | 0.67–2.12 | 0.5480 |
| Vascular invasion | |||||||||||||||
| Positive | 2.11 | 1.16–3.84 | 0.0151 | 1.53 | 0.82–2.86 | 0.1839 | 1.56 | 0.84–2.90 | 0.1636 | 1.73 | 0.93–3.21 | 0.0844 | 1.68 | 0.91–3.11 | 0.0983 |
| TNM stage | |||||||||||||||
| III+IV | 4.29 | 1.32–13.95 | 0.0154 | 3.41 | 1.02–11.46 | 0.0473 | 3.55 | 1.06–11.91 | 0.0406 | 3.52 | 1.04–11.88 | 0.0425 | 3.56 | 1.06–11.96 | 0.0405 |
| II | 2.25 | 0.67–7.58 | 0.1900 | 1.93 | 0.55–6.71 | 0.3017 | 1.96 | 0.57–6.81 | 0.2872 | 1.99 | 0.57–6.96 | 0.2792 | 1.93 | 0.55–6.74 | 0.3014 |
COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; LXR, liver X receptor; RXRα, retinoid X receptor α; SREBP-1c, sterol regulatory element binding protein-1c; HR, hazard ratio; CI, confidence interval; ref., hazard ratio reference value of 1; TNM, Tumor-Node-Metastasis.
Prognostic significance of combinations of COUP-TFII and LXR expression
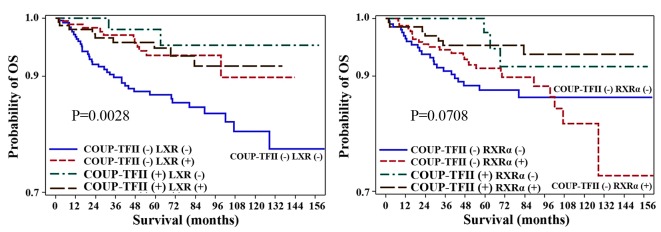
The aforementioned results indicated that COUP-TFII or LXR expression may be positive prognostic factors for patients with colorectal cancer. Therefore, the OS rate for patients with LXR- and COUP-TFII-positive immunostaining was compared with that of patients with LXR- and COUP-TFII-negative immunostaining. Patients with LXR- and COUP-TFII-positive immunostaining had a significantly higher OS rate than those with LXR- and COUP-TFII-negative immunostaining (P=0.0028; Fig. 3). Additionally, Cox proportional hazards regression analysis revealed that the negative expression of LXR and COUP-TFII was associated with a significantly worse prognosis (HR, 2.43; 95% CI, 1.17–5.04; P=0.0171) compared with the positive expression of LXR and COUP-TFII (Tables IV and VI).
Figure 3.
Association between combinations of COUP-TFII and LXR expression and survival of patients with colorectal cancer. Kaplan-Meier OS curves for 707 colorectal cancer patients, according to the expression levels of COUP-TFII and LXR in combination. OS rates are indicated in each panel. OS, overall survival; COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; LXR, liver X receptor.
Table VI.
Adjusted HRs for combinations of COUP-TFII and LXR expression.
| COUP-TFII + LXR | |||
|---|---|---|---|
| Characteristic | HR | 95% CI | P-value |
| C(−)L(−) | 2.33 | 1.11–4.89 | 0.0256 |
| C(−)L(+) | 1.15 | 0.47–2.80 | 0.7597 |
| C(+)L(−) | 0.54 | 0.12–2.50 | 0.4287 |
| C(+)L(+) | ref. | ||
| Sex | |||
| Male | 1.86 | 1.06–3.26 | 0.0299 |
| Age, years | |||
| <65 | 1.36 | 0.78–2.37 | 0.2828 |
| Grade | |||
| 3+4 | 1.84 | 0.70–4.82 | 0.2128 |
| 2 | 0.97 | 0.56–1.69 | 0.9240 |
| Tumor size, cm | |||
| ≥5 | 1.20 | 0.68–2.13 | 0.5227 |
| Vascular invasion | |||
| Positive | 1.38 | 0.74–2.60 | 0.3144 |
| TNM stage | |||
| III+IV | 3.28 | 0.98–11.02 | 0.0546 |
| II | 1.85 | 0.53–6.41 | 0.3338 |
COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; LXR, liver X receptor; RXRα, retinoid X receptor α; SREBP-1c, sterol regulatory element binding protein-1c; C, COUP-TFII; L, LXR; (−), negative expression; (+), positive expression; HR, hazard ratio; CI, confidence interval; ref., hazard ratio reference value of 1.
Discussion
The identification of biomarkers for predicting the prognosis of colorectal cancer will aid the adjustment of therapeutic strategies to individual patients. Cholesterol is a known risk factor for patients with colorectal cancer. There are several transcription factors involved in cholesterol homeostasis, including LXR, RXRα and SREBP-1c. The role served by LXR in carcinogenesis was investigated in several tumor types in previous studies (8–16). The tumor-protective actions of LXR were revealed in a previous study, which revealed that the ligand-induced activation of LXR or transfection with LXRα blocked entry into G1 phase, increased caspase-dependent apoptosis and slowed the growth of xenograft tumors in mice (7). Gene expression analysis revealed that the activation of LXRα affected lipid metabolic networks and increased cholesterol efflux in the intestine (7). However, to the best of our knowledge, the clinical significance of LXR expression in colorectal cancer has not been previously investigated.
In the present study, LXR expression was observed in 50.9% of colorectal cancer patients and was associated with favorable clinical outcomes, such as improved OS rates and lack of vascular invasion. However, it was not possible to discriminate between the expression of LXRα or β in the present study, as an anti-LXRα/β antibody was used. A future study will determine which type of LXR is more predictive of the prognosis in colorectal cancer. To the best of our knowledge, the present study is the first to demonstrate that LXR can be a positive prognostic factor for colorectal cancer, although there are several reports demonstrating that ligands of LXR inhibit cell proliferation in a number of cancer cell lines (8–14).
RXR has been implicated in cancer chemoprevention (17,18). However, the clinical significance of RXRα in colorectal cancer remains unknown. RXRα expression was observed in 56.4% of colorectal cancer patients in the present study and it was inversely associated with tumor grade. Further studies using RNA interference, or transfection with RXRα and LXR, are required to reveal the association between OS rates and LXR expression in patients with colorectal cancer.
No associations were found between expression of SREBP-1c and clinicopathological characteristics; this result is different from another study, in which the high expression of SREBP-1 predicted a poor prognosis for patients with pancreatic cancer (23). The different roles of SREBP-1c in cancer may depend on the tumor type.
In the present study, COUP-TFII expression was associated with an improved OS rate in a large cohort of patients with colorectal cancer with a long follow-up period. Our previous study revealed that COUP-TFII expression was not associated with lymph node metastasis or vascular invasion; this result may have been due to the relatively small number of patients with colorectal cancer who were included in the study (33). COUP-TFII expression was significantly negatively associated with vascular invasion and TNM stage; these results are similar to those of another study, which identified that high COUP-TFII transcript levels were associated with increased survival time, and that its expression inhibits the transforming growth factor-β (TGF-β)-dependent epithelial-mesenchymal transition (EMT) in breast cancer (36). In this study, the expression of TGF-β in patients with colorectal cancer was not examined. However, we hypothesize that there will be the downregulation of TGFβ in COUP-TFII-positive tumors. Our future study may investigate the expression of TGF-β and genes involved in EMT in patients with colorectal cancer.
Several studies have demonstrated that COUP-TFII is involved in cancer progression and metastasis (27,37,38). A recent study described the positive regulation of Snail1 by COUP-TFII, with the consequent downregulation of E-cadherin in colon cancer cell lines (37). The discrepancies between the results of the present study and those of Bao et al (37) may be due to other proteins associated with COUP-TFII in different cell lines, the sample size, and the genetic background of patients with colorectal cancer who were included in the studies. Although extensive studies have been performed recently (36–38), uncertainties remain concerning the role of COUP-TFII in cancer. Further studies using COUP-TFII-knockdown or overexpression are required to identify why the expression of COUP-TFII is negatively associated with TNM stage or vascular invasion in colorectal cancer.
Patients with LXR- and COUP-TFII-positive immunostaining had significantly better OS rates than those with LXR- and COUP-TFII-negative immunostaining. These data suggest that immunostaining for LXR and COUP-TFII in colorectal cancer samples at diagnosis may aid the prediction of the prognosis of patients with colorectal cancer.
The present study has limitations that should be noted. First, mortality during the study may have been too low to yield statistically significant data about whether TNM stage and vascular invasion were prognostic factors for patients in the study. Second, it was not demonstrated which type of LXR is a more important prognostic factor for colorectal cancer. Third, the molecular mechanisms responsible for the observed improvement in OS in patients with LXR-, or COUP-TFII-positive tumors have not been identified.
In summary, the present study demonstrated that LXR and COUP-TFII expression may be positive prognostic markers for patients with colorectal cancer. The results of the current study also suggest that the combined immunohistochemical examination of LXR and COUP-TFII expression in diagnostic samples of colorectal cancer may aid prognostic prediction. Future prospective and mechanistic studies evaluating the molecular interactions of LXR and COUP-TFII are required to confirm the findings of the present study.
Acknowledgements
The present study was supported by the National Research Foundation of Korea, funded by the Korean Government (grant no. 2016R1A5A2007009), and by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT & Future Planning (grant no. 2016R1C1B2007429).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 3.Mangeksdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKenna NJ, Cooney AJ, DeMayo FJ, Downes M, Glass CK, Lanz RB, Lazar MA, Mangelsdorf DJ, Moore DD, Qin J, et al. Minireview: Evolution of NURSA, the nuclear receptor signaling atlas. Mol Endocrinol. 2009;23:740–746. doi: 10.1210/me.2009-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuu CP, Kokontis JM, Hilpakka RA, Liao S. Modulation of liver X receptor signaling as novel therapy for prostate cancer. J Biomed Sci. 2007;14:543–553. doi: 10.1007/s11373-007-9160-8. [DOI] [PubMed] [Google Scholar]
- 6.Wójcicka G, Jamroz-Wisniewska A, Horoszewicz K, Beltowski J. Liver X receptors (LXRs). Part I: Structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dows (Online) 2007;61:736–759. [PubMed] [Google Scholar]
- 7.Lo Sasso G, Bovenga F, Murzili S, Salvatore L, Di Tullio G, Martelli N, D'Orazio A, Rainaldi S, Vacca M, Mangia A, et al. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology. 2013;144(1497–1507):e1–e13. doi: 10.1053/j.gastro.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Fukuchi J, Kokontis JM, Hiipakka RA, Chuu CP, Liao S. Antiproliferative effect of liver X receptor agonists on LNCaP human prostate cancer cells. Cancer Res. 2004;64:7686–7689. doi: 10.1158/0008-5472.CAN-04-2332. [DOI] [PubMed] [Google Scholar]
- 9.Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi J, Chen RY, Liao S. Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res. 2006;66:6482–6486. doi: 10.1158/0008-5472.CAN-06-0632. [DOI] [PubMed] [Google Scholar]
- 10.Pommier AJ, Alves G, Viennois E, Bernard S, Communal Y, Sion B, Marceau G, Damon C, Mouzat K, Caira F, et al. Liver X receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 11.Vedin LL, Lewandowski SA, Parini P, Gustafsson JA, Steffensen KR. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30:575–579. doi: 10.1093/carcin/bgp029. [DOI] [PubMed] [Google Scholar]
- 12.Uno S, Endo K, Jeong Y, Kawana K, Miyachi H, Hashimoto Y, Makishima M. Suppression of beta-catenin signaling by liver X receptor ligands. Biochem Pharmacol. 2009;77:186–195. doi: 10.1016/j.bcp.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Scoles DR, Xu X, Wang H, Tran H, Taylor-Harding B, Li A, Karlan BY. Liver X receptor agonist inhibits proliferation of ovarian carcinoma cells stimulated by oxidized low density lipoprotein. Gynecol Oncol. 2010;116:109–116. doi: 10.1016/j.ygyno.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Geyeregger R, Shehata M, Zeyda M, Kiefer FW, Stuhlmeier KM, Porpaczy E, Zlabinger GJ, Jäger U, Stulnig TM. Liver X receptors interfere with cytokine-induced proliferation and cell survival in normal and leukemic lymphocytes. J Leukoc Biol. 2009;86:1039–1048. doi: 10.1189/jlb.1008663. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and (p27)Kip, polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaschke F, Leppanen O, Takata Y, Caglayan E, Liu J, Fishbein MC, Kappert K, Nakayama KI, Collins AR, Fleck E, et al. Liver X receptor agonists suppress vascular smooth muscle cell proliferation and inhibit neointima formation in balloon-injured rat carotid arteries. Cir Res. 2004;95:e110–e123. doi: 10.1161/01.RES.0000150368.56660.4f. [DOI] [PubMed] [Google Scholar]
- 17.Fan YY, Spencer TE, Wang N, Moyer MP, Chapkin RS. Chemopreventive n-3 fatty acids activate RXRalpha in colonocytes. Carcinogenesis. 2003;24:1541–1548. doi: 10.1093/carcin/bgg110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang SY, Shen SR, Shyu RY, Yu JC, Harn HJ, Yeh MY, Lee MM, Chang YC. Expression of nuclear retinoid receptors in normal, premalignant and malignant gastric tissues determined by in situ hybridization. Br J Cancer. 1999;80:206–214. doi: 10.1038/sj.bjc.6690340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R, Shoemaker AR, Verma AK. Retinoic acid nuclear receptors and tumor promotion: Decreased expression of retinoic acid nuclear receptors by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Carcinogenesis. 1994;15:701–705. doi: 10.1093/carcin/15.4.701. [DOI] [PubMed] [Google Scholar]
- 20.Xu XC, Sozzi G, Lee JS, Lee JJ, Pastorino U, Pilotti S, Kurie JM, Hong WK, Lotan R. Suppression of retinoic acid receptor beta in non-small-cell lung cancer in vivo: Implications for lung cancer development. J Natl Cancer Inst. 1997;89:624–629. doi: 10.1093/jnci/89.9.624. [DOI] [PubMed] [Google Scholar]
- 21.Yang YA, Han WF, Morin PJ, Chrest FJ, Pizer ES. Activation of fatty acid synthesis during neoplastic transformation: Role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp Cell Res. 2002;279:80–90. doi: 10.1006/excr.2002.5600. [DOI] [PubMed] [Google Scholar]
- 22.Yang Yu, Morin PJ, Han WF, Chen T, Bomman DM, Gabrielson EW, Pizer ES. Regulation of fatty acid synthase expression in breast cancer by sterol regulatory element binding protein-1c. Exp Cell Res. 2003;282:132–137. doi: 10.1016/S0014-4827(02)00023-X. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, He W, Luo M, Zhou Y, Chang G, Ren W, Wu K, Li X, Shen J, Zhao X, Hu Y. SREBP1 regulates tumorigenesis and prognosis of pancreatic cancer through targeting lipid metabolism. Tumour Biol. 2015;36:4133–4141. doi: 10.1007/s13277-015-3047-5. [DOI] [PubMed] [Google Scholar]
- 24.Smirnov DA, Hou S, Liu X, Claudio E, Siebenlist UK, Ricciardi RP. COUP-TFII is up-regulated in adenovirus type 12 tumorigenic cells and is a repressor of MHC class I transcription. Virology. 2001;284:13–19. doi: 10.1006/viro.2001.0913. [DOI] [PubMed] [Google Scholar]
- 25.Navab R, Wang Y, Chow YH, Wang A, Jankov RP, Takamoto N, Tsai SY, Tsai MJ, Tanswell AK, Hu J. Regulation of human Clara cell 10 kD protein expression by chicken ovalbumin upstream promoter transcription factors (COUP-TFs) Am J Respir Cell Mol Biol. 2002;27:273–285. doi: 10.1165/rcmb.2002-0014OC. [DOI] [PubMed] [Google Scholar]
- 26.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 27.Navab R, Gonzalez-Santos JM, Johnston MR, Liu J, Brodt P, Tsao MS, Hu J. Expression of chicken ovalbumin upstream promoter-transcription factor II enhances invasiveness of human lung carcinoma cells. Cancer Res. 2004;64:5097–5105. doi: 10.1158/0008-5472.CAN-03-1185. [DOI] [PubMed] [Google Scholar]
- 28.Nakshatri H, Mendonca MS, Bhat-Nakshatri P, Patel NM, Goulet RJ, Jr, Cornetta K. The orphan receptor COUP-TFII regulates G2/M progression of breast cancer cells by modulating the expression/activity of p21(WAF1/CIP1), cyclin D1, and cdk2. Biochem Biophys Res Commun. 2000;270:1144–1153. doi: 10.1006/bbrc.2000.2562. [DOI] [PubMed] [Google Scholar]
- 29.Safe S, Jin UH, Hedrick E, Reeder A, Lee SO. Minireview: Role of orphan nuclear receptors in cancer and potential as drug targets. Mol Endocrinol. 2014;28:157–172. doi: 10.1210/me.2013-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litchfield LM, Klinge CM. Multiple roles of COUP-TFII in cancer initiation and progression. J Mol Endocrinol. 2012;49:R135–R148. doi: 10.1530/JME-12-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin J, Tsai SY, Tsai MJ. The critical roles of COUP-TFII in tumor progression and metastasis. Cell Biosci. 2014;4:58. doi: 10.1186/2045-3701-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boudot A, Le Dily F, Pakdel F. Involvement of COUP-TFs in cancer progression. Cancers (Basel) 2011;3:700–715. doi: 10.3390/cancers3010700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin SW, Kwon HC, Rho MS, Choi HJ, Kwak JY, Park JI. Clinical significance of chicken ovalbumin upstream promoter-transcription factor II expression in human colorectal cancer. Oncol Rep. 2009;21:101–106. [PubMed] [Google Scholar]
- 34.Lan YT, Yang SH, Chang SC, Liang WY, Li AF, Wang HS, Jiang JK, Chen WS, Lin TC, Lin JK. Analysis of the seventh edition of American Joint Committee on colon cancer staging. Int J Colorectal Dis. 2012;27:657–663. doi: 10.1007/s00384-011-1366-6. [DOI] [PubMed] [Google Scholar]
- 35.Hsu FD, Nielsen TO, Alkushi A, Dupuis B, Huntsman D, Liu CL, van de Rijn M, Gilks CB. Tissue microarrays are an effective quality assurance tool for diagnostic immunohistochemistry. Mod Pathol. 2002;15:1374–1380. doi: 10.1097/01.MP.0000039571.02827.CE. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Han Y, Huang H, Qu L, Shou C. High NR2F2 transcript is associated with increased survival and its expression inhibits TGF-β-dependent epithelial-mesenchymal transition in breast cancer. Breast Cancer Res Treat. 2014;147:265–281. doi: 10.1007/s10549-014-3095-3. [DOI] [PubMed] [Google Scholar]
- 37.Bao Y, Gu D, Feng W, Sun X, Wang X, Zhang X, Shi Q, Cui G, Yu H, Tang C, Deng A. COUP-TFII regulates metastasis of colorectal adenocarcinoma cells by modulating Snail1. Br J Cancer. 2014;111:933–943. doi: 10.1038/bjc.2014.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bringuier PP, Schalken JA, Hervieu V, Giroldi LA. Involvement of orphan nuclear receptor COUP-TFII in cadherin-6 and cadherin-11 regulation: implications in development and cancer. Mech Dev. 2015;136:64–72. doi: 10.1016/j.mod.2015.02.001. [DOI] [PubMed] [Google Scholar]