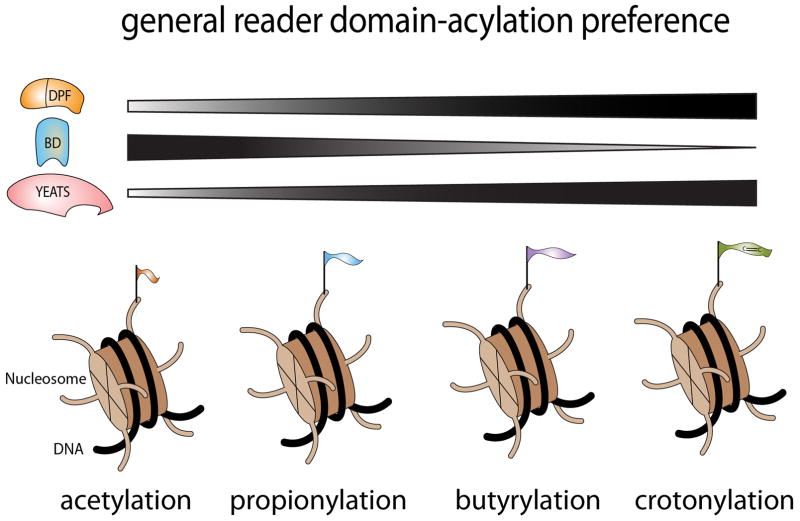
Figure 1. General preferences of histone readers for the distinct histone lysine acylation states.
Recent studies have uncovered the presence of additional lysine acylation states in histones, including lysine propionylation, butyrylation, crotonylation, β-hydroxybutyrylation, succinylation and malonylation. Investigators are now unveiling how these distinct acylation “flavors” are “read” or interpreted in the context of chromatin. New studies from the Li and Kutateladze groups have added to the list of reader domains that bind the bulkier histone acyl forms that are largely “off limits” to most bromodomains (BD). In this issue of Structure, Kutateladze et al. define the structural basis of the interaction of H3K14bu with the double PHD finger (DPF) of MORF, thus contributing to the finding that the DPF domain is a novel reader of lysine acylation. DPF domains, similar to YEATS domains, largely prefer longer acyl forms of lysines. It will be of great interest to determine how DPF domains contribute to transcriptional regulation through their ability to be recruited to lysine acylation events in chromatin.