Fig. 1.
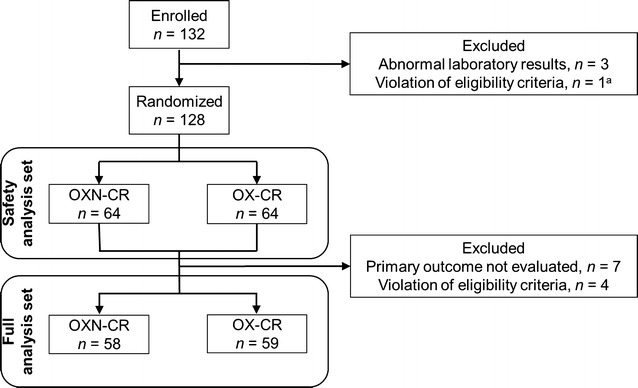
Flowchart of patients with cancer-related pain who were randomized to receive either controlled-release oxycodone/naloxone (OXN-CR) or controlled-release oxycodone (OX-CR). aThe patient was found to have significant structural/functional abnormalities of the gastrointestinal tract which was deemed to be not appropriate for oral medicine administration
