Fig. 6.
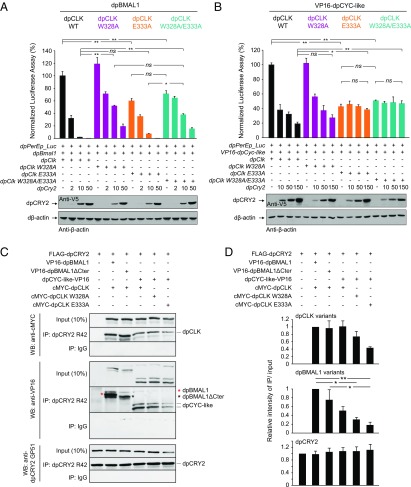
The dpCLK W328 and E333 residues independently contribute to TAD-dependent and TAD-independent repression by dpCRY2. (A and B) Effects of dpCLK mutations (dpCLK W328A, dpCLK E333A, and dpCLK W328A/E333A) in the presence (A) or absence (B) of the dpBMAL1 C terminus. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence (+) of dpBMAL1 (A) or VP16–dpCYC-like (B) and dpCLK variants expression plasmids (5 ng each) with increasing doses of dpCRY2; amounts are given in nanograms. Firefly luciferase activity was computed relative to renilla luciferase activity. Each value is the mean ± SEM of three replicates. Western blots of V5-tagged dpCRY2 and Drosophila β-actin protein expression levels are shown below the graphs. *P < 0.05; **P < 0.01; ns, not significant (two-way ANOVA followed by Tukey’s post hoc test). (C) Coimmunoprecipitations (IP) of c-Myc–dpCLK and either VP16-dpBMAL1 or VP16-dpBMAL1ΔCter and of dpCYC-like–VP16 and c-Myc–dpCLK WT, c-Myc–dpCLK W328A, or c-Myc-dpCLK E333A by FLAG-dpCRY2 with anti-dpCRY2 R42 antibody from transfected S2 cells. Western blots (WB) were performed using the indicated antibodies. The double band for dpCYC-like–VP16 likely corresponds to alternatively used translation initiation sites. The top band was quantified in D. (D) Quantification of C. For each protein, the relative intensity corresponds to the intensity of IP over input signal measured using Image J in each condition, relative to the intensity of IP over input signal of dpCRY2, WT dpCLK, and WT dpBMAL1. Each value is the mean ± SEM of three independent experiments. dpCLK variants, P < 0.05; dpBMAL1 variants, P < 0.005; dpCRY2, P = 0.96 (one-way ANOVA). *P < 0.05; **P < 0.01 (Tukey’s post hoc test).