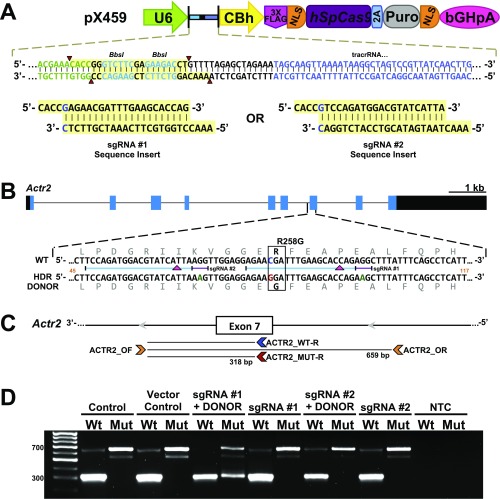
Fig. S2.
Strategy to generate the independent Actr2 knockin (Actr2G) allele by CRISPR/Cas9 technology. (A) Schematic representation of the pX459 vector and the designed guide sequence inserts. The image is an adaptation from Ran et al. (55). The guide oligos contain overhangs for ligation into the pair of BbsI sites (highlighted in yellow) in pX459. Digestion of pX459 with BbsI allows the replacement of the type II restriction sites (red arrowheads) with direct insertion of annealed oligos. Likewise, a G-C base pair (blue type in sgRNA insert sequences) is added at the 5′ end of the guide sequence for U6 transcription. Color coding of the sequence correlates with positions within the guide RNA expression cassette located in the pX459 vector (green type is the 3′ end of the U6 promoter; bright blue type on yellow highlighting identifies BbsI sites; black type is the chimeric guide RNA backbone; and blue type is the tracrRNA sequence. (B) sgRNA and HDR donor design for targeting the mouse Actr2 locus for R258G incorporation. The Actr2 gene is shown in the forward direction. Black and blue filled boxes indicate exons and the ORF, respectively. Gray type indicates the in-frame amino acid sequence; blue and red type indicate the C→G nucleotide change resulting in the R258G substitution; green type indicates synonymous mutations to block subsequent CRISPR binding following sequence replacement. Numbers in orange indicate the position within the 161-bp HDR template that is depicted in the figure. The blue and purple bars indicate the sgRNA and PAM, respectively, for sgRNA #1 and #2, and red arrowheads indicate the DSB position within Actr2 for each guide. (Scale bar for the Actr2 locus, 1 kb; intronic regions are reduced for fit.) (C) Schematic representation of Actr2 primer locations. Note reverse orientation. HDR-mediated DNA integration within the Actr2 gene was tested in the N2a cell line using the two sgRNAs and their respective ssDNA donor sequences for HDR-mediated knockin (Table S4). (D) Genotyping of heterogeneous N2a cell populations after puromycin selection. PCR was performed as described in Materials and Methods and depicted in C. Mut and Wt indicate competitive PCR with primers ACTR_OF/R and MUT-R or WT-R, respectively; NTC, no template control. In postselected heterogeneous N2a cells, the 318-bp band indicating the presence of the Actr2G allele within the heterogeneous cell population was observed when PCR was performed with the mutant-specific primer (Mut) on gDNA isolated from cells cotransfected with sgRNA #1 + DONOR.