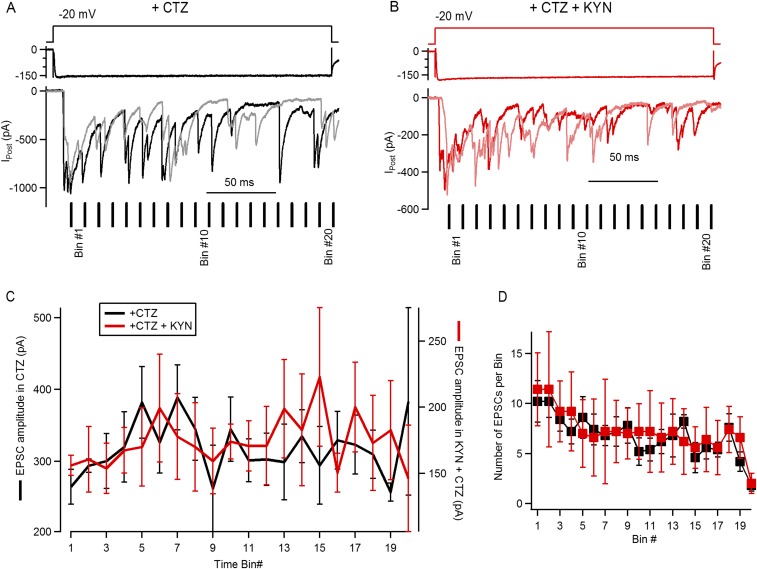
Fig. S2.
Determining saturation of postsynaptic receptors during maximal stimulation of the IHC ribbon synapse. (A) Representative synaptic responses to step depolarizations in the presence of CTZ: schematics of the voltage pulse (Top), IHC Ca2+ current (Middle), and two individual postsynaptic currents (Bottom). The 10-ms time bins, used to analyze amplitudes (C), are indicated for this specific example. (B) Same as A, but for two recordings obtained with CTZ and KYN, 1 mM (KYN). (C) Average amplitude of EPSCs occurring within each time bin for events obtained during superfusion of CTZ (left axis) or both CTZ and KYN (right axis). (D) Number of EPSCs obtained per time bin, per recording. These results suggest that a steady decay in Ca2+ current amplitude has limited effect on the kinetics of the postsynaptic response depression, specifically on the fast phase of decay. With further reduction in the Ca2+ influx toward the end of the stimulus, release shows a stronger decay and significantly lower responses to square pulses. Some evidence indicates that CTZ not only reduces desensitization of AMPA receptors but could also produce a left shift in the agonist dose–response curve [Patneau et al. (29)]. This observation raises the possibility that at the onset of the postsynaptic response, when maximal release occurs, CTZ could produce an artificial saturation of the receptors, and this in turn alters the decay parameters established in Fig. 4. We decided to evaluate saturation of postsynaptic receptors during the superfusion of CTZ, using the competitive antagonist KYN that partially blocks AMPA responses and potentially relieves saturation. Step depolarizations to –20 mV were applied on the IHC in the presence of CTZ (100 μM), similarly to Fig. 4A. We reasoned that if the postsynaptic response was saturated at the initial peak, EPSCs occurring shortly after this should present a smaller average amplitude compared with later ones. We took advantage of the release mode at this ribbon synapse, in which steady stimulation produces the activation of multiple EPSCs [Keen and Hudspeth (20); Goutman and Glowatzki (21)]. The amplitude of individual EPSCs was measured and then averaged in 10-ms time bins, considering t = 0 when the peak occurred (i.e., EPSCs occurring at the peak were excluded of the analysis) (see A). In CTZ, EPSCs that occurred at bin 1 (first 10 ms after the peak) presented an average amplitude of 264 ± 24 pA (n = 5 recordings, six to nine repetitions each, 4–17 EPSCs each) (C). Considering that the peak response obtained in this set of experiments in CTZ was 774 ± 61 pA (n = 5 recordings), the average EPSC size represented ∼34% of the maximal response. The amplitude in the following bins increased up to bin 5, with an average of 382 ± 50 pA, ∼49% of the peak (n = 5 recordings, six to nine repetitions, 2 to 14 EPSCs). We also calculated the average amplitude of the last 10 time bins, obtaining a value of 313 ± 10 pA (n = 5). None of these differences were statistically significant (ANOVA, P = 0.08). As shown in B, the same stimulation protocol was repeated with the addition of 1mMKYN (+CTZ). As expected, the maximal response was reduced to 403 ± 63 pA, representing a 49 ± 5% reduction compared with responses obtained in CTZ (n = 5 recordings, P < 0.001, t test). We also measured EPSC amplitudes and averaged them in 10-ms time bins. Interestingly, having partially blocked postsynaptic receptors, a similar profile was observed in EPSC sizes, with an average amplitude of 156 ± 17 pA in bin 1 (n = 5 recordings, six to nine repetitions each, 6–16 EPSCs), rising to 199 + 91 pA at bin 6 (3–11 EPSCs), and with an average amplitude of 180 ± 7 pA for the last 10 bins (ANOVA, P = 0.95). The amplitude of the first bin represented ∼39% of the corresponding peak response in CTZ + KYN, whereas bin 6 was ∼49%. The average number of EPSCs obtained in each time bin (over repetitions in each recording) is compared in D. The number of EPSCs decreased on later time bins, but no differences were found between drug treatments (two-way ANOVA, P < 0.001 for bin number and P = 0.72 for drugs). Results indicate that saturation of AMPA receptors during the peak of the postsynaptic response would be very limited and that the effect of CTZ on the decay kinetics should be mostly attributed to the desensitization removal.