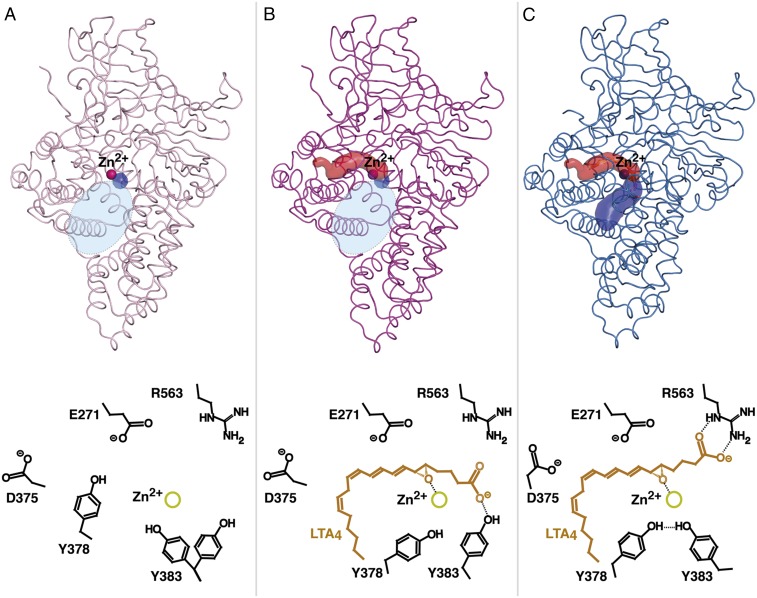
Fig. 4.
Tunnel formations (Upper; the structures and corresponding Zn2+ are colored as in Fig. 3 B and C) and proposed mechanism of substrate binding (Lower) during dynamic domain motion between open and closed D375N. (A) In the open conformation (polar part of binding pocket is shown as tiny blue spot; wide space between domains at the entrance of the active site is indicated as an oval in shaded light blue) Y378 blocks the entrance of the hydrophobic cavity. (B) During substrate binding, lid region moves and opens the hydrophobic tunnel (red surface representation) for LTA4 entrance. When LTA4 slides into the hydrophobic pocket, its carboxylate group is guided by Y383. (C) During domain motion, the ω-end reaches the bottom of the cavity, the epoxide binds to Zn2+, and the C1 carboxylate switches from Y383 to R563. The closed conformation affords productive substrate alignment and allows epoxide activation and subsequent hydrolysis according to an SN1 mechanism. The polar arm of the binding pocket that is formed as a result of convergence of domains is shown as blue surface.