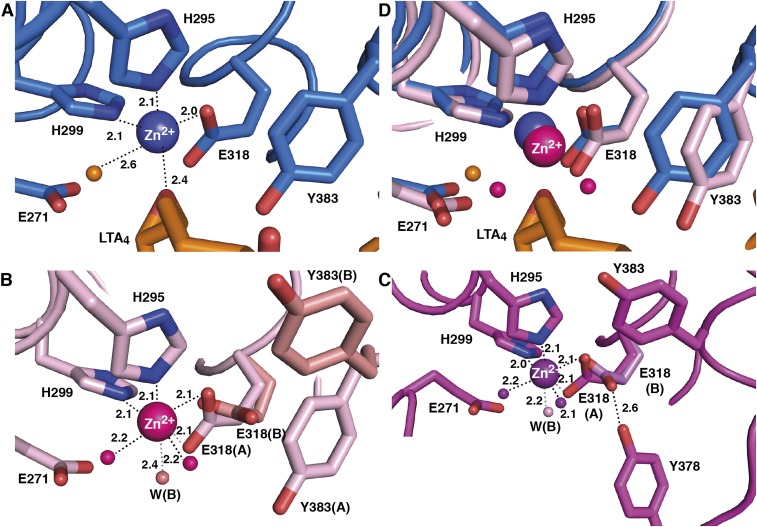
Fig. S4.
Geometry of Zn2+-binding site in open and closed conformations of LTA4H. (A) Monodentate carboxylate group coordination in the closed D375N-LTA4 (residues and Zn2+ are in blue, LTA4 and catalytic water are in orange). Distances are labeled in Å. (B) Bidentate carboxylate group coordination in open D375N (residues, Zn2+ and coordinating waters are in pink) with blocked tunnel and (C) in open D375N (residues, Zn2+ and coordinating waters are in magenta) with open tunnel. Alternative conformation of residues and water molecules in open D375N (B) and D375N (C) are colored salmon and light magenta, respectively. (D) Superposition of Zn2+-binding site in open and closed conformations of D375N.