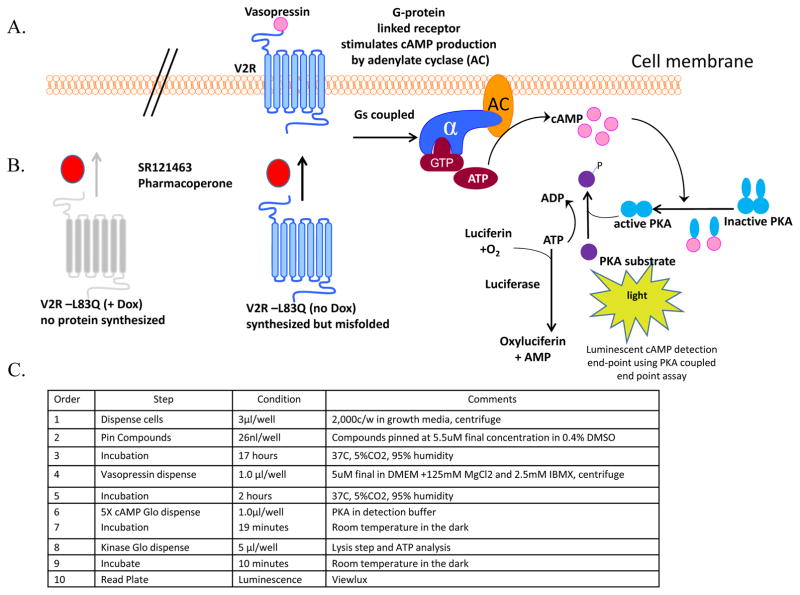
Figure 1. Schematic diagram of the V2R cAMP detection luminescence reporter assay.
A. Representation of a cell-based assay to monitor pharmacoperone rescue of mutant vasopressin receptor function. In wild type V2R responses, vasopressin initiates Gαs activation of adenylate cyclase which increases the concentration of cAMP. In this detection format cAMP binds to protein kinase A, and the regulatory subunits undergo a conformational change to release the catalytic subunits. The free catalytic subunits then catalyze the transfer of the terminal phosphate of ATP to a protein kinase A substrate, consuming ATP in the process. The level of remaining ATP is determined using the luciferase-based ATP detection reagent. Luminescence is inversely proportional to cAMP levels. Thus, as cAMP concentration increases, luminescence decreases. B. Untreated cells “no Dox” have an active tet promoter resulting in expression of misfolded mutant protein. Upon treatment of the cells with vasopressin the pharmacoperone (SR121463) receptor folding is corrected and function is rescued, resulting in a Gs-coupled response which is readily monitored. In “+ Dox” cells, the Tet minus promoter is inactive and no protein is made, thus cells are non-responsive to SR121463 and V2R; i.e., the counterscreen. C. V2R uHTS assay protocol in 1,536 well plate format.