Abstract
Context:
Methylene blue is an inhibitor of guanylate cyclase and hence prevents vasoplegia mediated by nitric oxide in patients with sepsis.
Aims:
This study aimed to analyze the effect of methylene blue on blood pressure maintenance following induction of anesthesia in patients presenting with peritonitis.
Subjects and Methods:
Thirty patients diagnosed to have perforation peritonitis were randomized into two groups (Group MB, Group NS). Patients in Group MB were given injection methylene blue 2 mg/kg over 20 min and patients in Group NS were given 50 ml of normal saline over 20 min, before induction. Heart rate, mean arterial pressure (MAP), cardiac output, and systemic vascular resistance (SVR) were recorded every 5 min for 1 h after infusion.
Statistical Analysis:
Hemodynamic parameters were analyzed using repeated-measures analysis of variance with Bonferroni's test. Blood gas analysis was analyzed using independent Student's t-test, and P < 0.05 was considered statistically significant.
Results:
MAP was lower at all-time points in Group NS than Group MB; however, it was statistically significant immediately, and 5 min the following induction. MAP fell from 94.8 ± 11.8 mmHg to 89.2 ± 16.0 mmHg immediate postinduction in Group MB and from 92.1 ± 9.8 mmHg to 74.1 ± 12.6 mmHg in Group NS. MAP and SVR were significantly higher in Group MB, 5 min following induction. No adverse events attributable to methylene blue were noted.
Conclusions:
Methylene blue contributes to the maintenance of postinduction hemodynamic stability in patients with perforation peritonitis.
Keywords: Methylene blue, peritonitis, sepsis
INTRODUCTION
Sepsis is a major cause of morbidity and mortality in critically ill patients and is associated with several derangements involving multiple different organs and systems. The principle hemodynamic changes in septic shock involve peripheral arterial vasodilatation, high cardiac output (CO), hypotension, and reduced tissue perfusion.[1] Nitric oxide (NO) plays a key role in the pathogenesis of septic shock.[2] Endotoxins and various cytokines like interferon and tumor necrosis factor increase the activity of inducible NO synthase (iNOS), resulting in increased levels of NO in patients with sepsis. NO and NO − related compounds are responsible for vascular hypocontractility and cellular damage. NO binds to guanylate cyclase, a smooth muscle enzyme and accelerates the conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP), which in turn mediates smooth muscle relaxation.[3] Methylene blue has direct inhibitory effects on NOS. It further prevents the accumulation of cGMP by blocking the activity of NO-dependent guanylate cyclase,[3] thus antagonizing the NO-mediated vasodilatation associated with sepsis.[4] It also reduces sepsis-induced negative cardiac inotropism. In addition, methylene blue is also known to restore the vascular reactivity to endogenous catecholamines.
In this study, we evaluated the effects of methylene blue infusion on the hemodynamics of patients with peritonitis undergoing laparotomy under general anesthesia.
SUBJECTS AND METHODS
This was a randomized controlled study conducted in our institute between August 2011 and August 2013. This study was conducted after approval from the Institute Research and Ethics Committee and written informed consent from participants was obtained. This study was registered with the Clinical Trials Registry of India (CTRI/2015/10/006256).
Thirty consecutive patients in the age group of 18–50 years, diagnosed to have peritonitis by clinical and radiological examination, who were hemodynamically stable and with the American Society of Anesthesiologists Class IIIE were recruited. Patients with known allergy to methylene blue, patients on anti-psychiatric drugs and patients with renal or liver failure were excluded from the study. All patients included in the study were randomly allocated into two groups by sealed envelope technique. Group MB (n = 15) received injection methylene blue infusion and Group NS (n = 15) received normal saline infusion [Figure 1].
Figure 1.

CONSORT diagram
Preoperative assessment included hemodynamic parameters (pulse rate, blood pressure, and oxygen saturation [SaO2]), baseline blood sugar, blood urea, serum creatinine, serum electrolytes, total bilirubin, and blood gas analysis.
In the operation theater, the patient was connected to monitors and baseline values of heart rate, noninvasive blood pressure, and SaO2 were obtained. An arterial line was secured under local anesthesia for invasive blood pressure monitoring (systolic blood pressure [SBP], mean arterial blood pressure [MAP]). The right internal jugular vein was cannulated with a 7 Fr triple lumen central venous catheter and baseline central venous pressure (CVP) was measured. Baseline CO and systemic vascular resistance (SVR) were obtained using a cardiac monitor (Vigileo/FloTrac™, Edwards Lifesciences, USA). Fluid preloading was started.
Patients in Group MB received injection methylene blue infusion (2 mg/kg) intravenously over 20 min. Patients in Group NS received normal saline infusion over the same duration. A standardized rapid sequence induction was performed with injection thiopentone, injection fentanyl, and injection succinylcholine. After tracheal intubation, mechanical ventilation was initiated and adequate plane of anesthesia was maintained with oxygen and nitrous oxide mixture, isoflurane to achieve a minimum alveolar concentration of 0.8–1 and muscle relaxation was maintained with a nondepolarizing muscle relaxant, atracurium.
Pulse rate, invasive blood pressure, CO, and SVR were recorded at the end of infusion, immediately after induction, 5 min after induction, and repeated every 5 min for the first 1 h. Subsequently, they were measured every 15 min, till the completion of surgery. Vasopressors were added if SBP and/or MAP dropped below 90 and 65 mmHg, respectively, or SBP and/or MAP fell below 20% even after fluid therapy.
At the end of the surgery, the standard extubation protocol included ensuring hemodynamic stability, reversal of neuromuscular blockade and monitoring for adequate respiratory efforts. A decision for elective mechanical ventilation was made if respiratory efforts were inadequate or patient was hemodynamically unstable. All the patients were shifted to the critical care unit postoperatively and monitored for a minimum period of 24 h. Patients were followed up for 7 days to record any adverse events.
Statistical analysis
Data collected were analyzed using SPSS statistical software version 16 (IBM, USA). Hemodynamic parameters were analyzed using repeated measures analysis of variance and Bonferroni's test for post hoc significance. Student's t-test was used to analyze blood gas values. P < 0.05 was considered statistically significant.
RESULTS
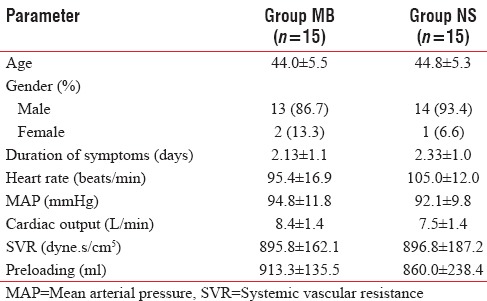
Thirty patients who were admitted to the Surgical Department with perforation peritonitis between August 2011 and August 2013 were taken up for the study after obtaining informed consent. The physical characteristics were similar in both the groups [Table 1].
Table 1.
Baseline parameters (mean±standard deviation)
Baseline heart rate and MAP values were comparable in both the groups [Figure 2]. MAP was significantly lower in Group NS than Group MB in the immediate postinduction period (74.13 ± 12.60 mmHg vs. 86.60 ± 17.80 mmHg, P < 0.05) and 5 min after induction (73.20 ± 07.78 mmHg vs. 83.00 ± 12.74 mmHg, P < 0.05). At all other time points, the MAP was significantly more in Group MB compared to Group NS, although not statistically significant [Figure 3].
Figure 2.
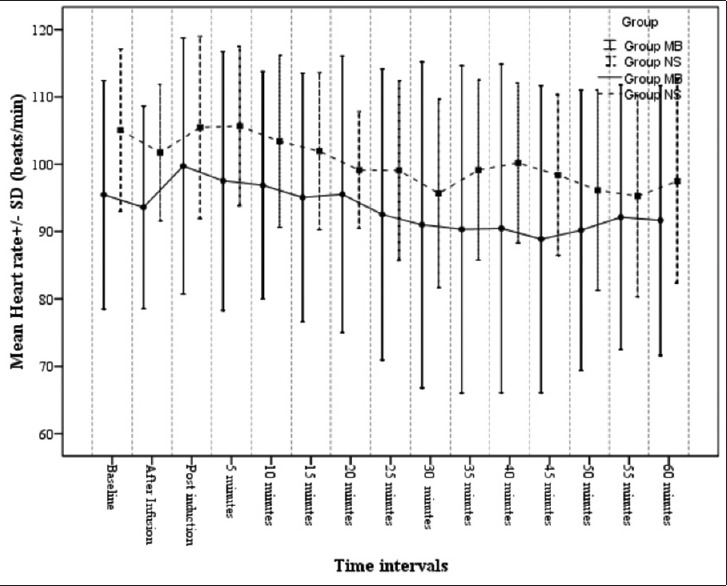
Heart rate variation in the study groups
Figure 3.
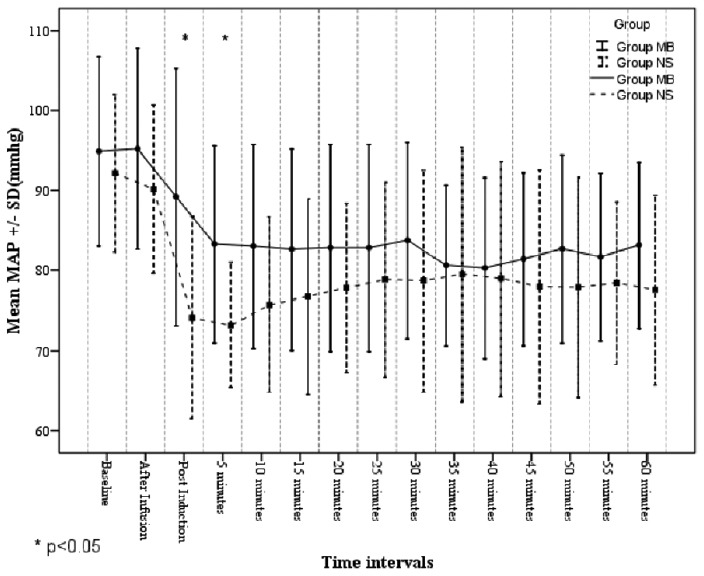
Mean arterial pressure in the study groups
There was a statistically significant difference in SVR between Group MB and Group NS, 5 min after induction. Baseline SVR was significantly higher in the Group MB at 5 min after induction (888.8 ± 162.5 dyne.s/cm5 Vs 767.2 ± 122.3 dyne.s/cm5, P < 0.05). At all other time points, SVR was higher in Group MB than Group NS, though not statistically significant [Figure 4].
Figure 4.
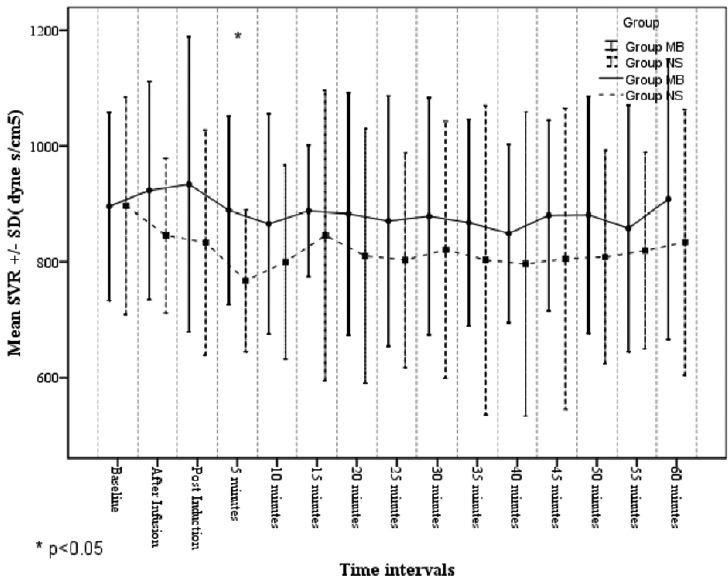
Systemic vascular resistance in the study groups
CO was comparable in both the groups [Figure 5]. In Group MB, 2 out of 15 (13.3%) patients required inotropic support. Both patients required injection dopamine infusion. In Group NS, 4 out of 15 (26.6%) patients required inotropic support. Two patients were given both injection dopamine, injection norepinephrine infusion, whereas the other two patients required only injection dopamine infusion.
Figure 5.
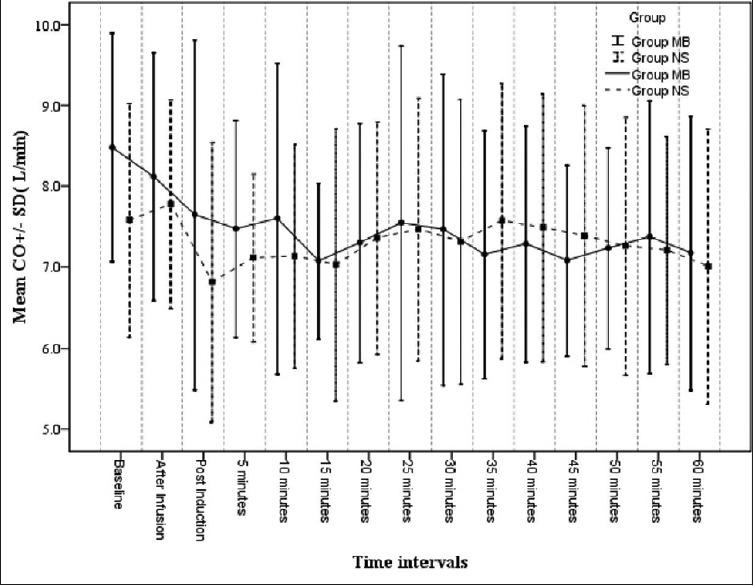
Cardiac output in the study groups
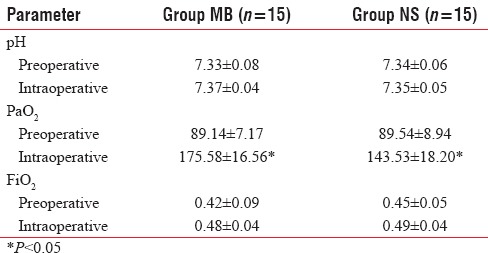
Baseline pH, PaO2 and FiO2 requirement were comparable in preoperative period. PaO2 was significantly more in Group MB compared to Group NS during the intraoperative period. pH and FiO2 requirement were not statistically significant during the intraoperative period [Table 2]. One patient in Group MB (6.6%) and three patients (20%) in Group NS required postoperative mechanical ventilation.
Table 2.
Comparison of pre- and intra-operative blood gas analysis
DISCUSSION
Septic shock is characterized by a severe inflammatory response that results in loss of vascular tone. This loss of vascular tone is mediated by the local and systemic release of a multitude of factors, kinins, and other acute phase reactants. Among these, the most important is the activation of guanylate cyclase and iNOS.[2] NO is responsible for smooth muscle relaxation through a cGMP pathway. Septic shock, therefore, is characterized by circulatory failure with low SVR, MAP, systemic hypoperfusion, and tissue malperfusion.
The main strategy in the management of sepsis is early goal-directed therapy.[1] One of the most important goals in this bundle is maintaining the MAP above 65 mmHg. A subgroup of patients fail to respond even to high doses of catecholamines and the mortality rates in these groups exceeds 40%. There is clearly a need for alternate or additional therapy. Recognition of NO as a mediator of sepsis-induced myocardial depression as well as an inhibitor of blood vessel response to vasopressors[1] is a possible first step in identifying an alternative intervention to septic shock.
iNOS prevents or corrects hypotension. Methylene blue blocks guanylate cyclase and thereby prevents NO-mediated vasodilation.[5] In patients who remain hypotensive despite administration of multiple agents, several studies and case reports have proven that methylene blue represents an alternate method to restore vascular tone and improve perfusion. However, the use of methylene blue in the treatment of septic shock is not considered a standard of care and often remains a therapy of last resort.
Most of the patients in sepsis due to hollow visceral organ perforation and peritonitis need to undergo laparotomy under anesthesia. Induction of anesthesia itself is known to cause a fall in blood pressure. There will be loss of sympathetic tone resulting in lowering of SVR. While this might be of little consequence in hemodynamically stable patients, it might be detrimental in patients with sepsis and septic shock. Inadequately prepared patients are, therefore, at risk of cardiac arrest on induction. There are no studies that show the effect of methylene blue in maintaining blood pressure following induction of anesthesia.
This study was designed to assess the effect of methylene blue on postinduction fall of blood pressure in patients with sepsis. Thirty patients presenting to the emergency department at JIPMER with sepsis due to hollow visceral organ perforation were included in the study. They were randomized to receive either methylene blue or normal saline (placebo) just before induction. These patients received fluid resuscitation preoperatively. Heart rate, systolic, diastolic and MAPs, CO, SVR, CVPs were monitored every 5 min for 1 h postinduction.
Our study showed a statistically significant difference in the MAPs between the groups at immediate postinduction and 5 min after induction, with these values being higher in Group MB, due to an increase in SVR. Several studies have shown the similar results. Donati et al.[6] found a progressive increase in MAP, SVR following administration of methylene blue. They recorded statistically significant increase in the pulmonary arterial pressure. In this study, we had not monitored pulmonary arterial pressures.
In this study, we found that the anti-vasodilatory effect of MB was not followed by an increase in CO due to increase in SVR. This is in accordance with other studies. Park et al.[7] found that there was no change in cardiac index after methylene blue infusion. Weingartner et al.[8] also found an increase in the left ventricular stroke work index suggesting an improvement in the cardiac status. This is probably due to the suppression of the effects of NO induced myocardial depression.
Our study found a transient increase in the PaO2 and PaO2/FiO2 ratio. This is in contrast to several studies which found a drop in PaO2/FiO2 ratio. Weingartner et al.[8] had shown that there was a decrease in PaO2/FiO2 ratio. This was particularly noticed in patients with severe lung injury. Since the design of our study was a preliminary one, we excluded patients with severe lung injury and unstable hemodynamics. This may explain the absence of a drop in the ratio in our data. However, the data on oxygen delivery is controversial.[9,10]
Kirov et al.[11] had shown that the use methylene blue in patients with sepsis reduced the inotropic requirement. In our study, 2 (13.3%) out of 15 patients in Group MB and 4 (26.6%) out of 15 patients required inotropic support. In Group MB both patients required only injection dopamine infusion, whereas in Group NS two patients required both injection dopamine and injection norepinephrine infusion. Other two patients in Group NS required only injection dopamine infusion. In addition to reduced requirement of inotropic support, we were able to wean the patients off inotropic support early in Group MB. In Group MB, 1 (6.6%) out of 15 patients required mechanical ventilation. In Group NS, 3 (20%) out of 15 patients required mechanical ventilation. There was no mortality in the study population.
Jaskille et al.[12] found that methylene blue succesfully reversed refractory vasoplegia after severe burns. In a meta-analysis of randomized trials on the use of noncatecholamine vasopressors in vasodilatory shock with the end point as mortality outcome, the authors report that while there are articles on the use of methylene blue for several types of vasodilatory shock, its use still remains a controversial therapeutic approach with unproven benefit.[13] Another meta-analysis of randomized clinical trials on the use of methylene blue in hypotensive patients with the primary aim of increasing MAPs was performed in 2013. This identified five randomized controlled trials and concluded that methylene blue increases the MAPs with no detrimental effect on survival.[14]
Another controversial issue is the timing of methylene blue administration in vasodilatory shock. Blacker and Whalen, in their report of a vasoplegic syndrome on cardiopulmonary bypass, reported the triphasic time-dependent alteration in NO synthesis. They identified the first 8 h as the period of highest NO and guanylate cyclase synthesis.[15] Many authors have opined that this period may be an appropriate therapeutic window in humans for administering methylene blue. Based on these findings, it is suggested that methylene blue should be administered early in the vasodilatory and vasoplegic phase. Methylene blue was used in treating vasoplegia following cardiopulmonary bypass.[16,17] It was used in patients with refractory vasoplegia following liver transplantation.[18]
Several studies and reports have demonstrated a favorable side effect profile for methylene blue. It may rarely cause tremors, shortness of breath, bluish discoloration of body fluids, interference with pulse oximetry readings, and acute hemolytic anemia in doses exceeding 40 mg/kg. A potentially fatal complication of serotonergic syndrome may occur when administered to patients on monoamine oxidase inhibitors and serotonergic agents.[19]
This study was designed as a pilot study for the use of methylene blue in patients with septic shock at our center. Therefore, patients were carefully selected to ensure patient safety during this study. Patients with hemodynamic instability and organ injury were excluded. This study aimed only at evaluating the role of methylene blue in preventing a fall in blood pressure at induction of anesthesia. Our data substantiate the claim that methylene blue can ameliorate postinduction fall in blood pressure, without any significant adverse events. Further studies are needed to confirm our findings in a larger group of patients and those with hemodynamic instability.
CONCLUSIONS
Our study concludes that methylene blue prevents fall in blood pressure during induction of anesthesia in hemodynamically stable patients with peritonitis. It also suggests a role for methylene blue in maintaining better MAP and in reducing the need for inotropic and ventilatory support in these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Zhang H, Szabo C, Preiser JC. Effects of nitric oxide in septic shock. Am J Respir Crit Care Med. 2000;161:1781–5. doi: 10.1164/ajrccm.161.6.9812004. [DOI] [PubMed] [Google Scholar]
- 3.Miclescu A, Wuklund L. Methylene blue, an old drug with new indications. J Rom Anesth Ter Int. 2010;17:35–41. [Google Scholar]
- 4.Preiser JC, Lejeune P, Roman A, Carlier E, De Backer D, Leeman M, et al. Methylene blue administration in septic shock: A clinical trial. Crit Care Med. 1995;23:259–64. doi: 10.1097/00003246-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Daemen-Gubbels CR, Groeneveld PH, Groeneveld AB, van Kamp GJ, Bronsveld W, Thijs LG. Methylene blue increases myocardial function in septic shock. Crit Care Med. 1995;23:1363–70. doi: 10.1097/00003246-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Donati A, Conti G, Loggi S, Münch C, Coltrinari R, Pelaia P, et al. Does methylene blue administration to septic shock patients affect vascular permeability and blood volume? Crit Care Med. 2002;30:2271–7. doi: 10.1097/00003246-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Park BK, Shim TS, Lim CM, Lee SD, Kim WS, Kim DS, et al. The effects of methylene blue on hemodynamic parameters and cytokine levels in refractory septic shock. Korean J Intern Med. 2005;20:123–8. doi: 10.3904/kjim.2005.20.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weingartner R, Oliveira E, Oliveira ES, Sant’Anna UL, Oliveira RP, Azambuja LA, et al. Blockade of the action of nitric oxide in human septic shock increases systemic vascular resistance and has detrimental effects on pulmonary function after a short infusion of methylene blue. Braz J Med Biol Res. 1999;32:1505–13. doi: 10.1590/s0100-879x1999001200009. [DOI] [PubMed] [Google Scholar]
- 9.Andresen M, Dougnac A, Díaz O, Hernández G, Castillo L, Bugedo G, et al. Use of methylene blue in patients with refractory septic shock: Impact on hemodynamics and gas exchange. J Crit Care. 1998;13:164–8. doi: 10.1016/s0883-9441(98)90001-6. [DOI] [PubMed] [Google Scholar]
- 10.Gachot B, Bedos JP, Veber B, Wolff M, Regnier B. Short-term effects of methylene blue on hemodynamics and gas exchange in humans with septic shock. Intensive Care Med. 1995;21:1027–31. doi: 10.1007/BF01700666. [DOI] [PubMed] [Google Scholar]
- 11.Kirov MY, Evgenov OV, Evgenov NV, Egorina EM, Sovershaev MA, Sveinbjørnsson B, et al. Infusion of methylene blue in human septic shock: A pilot, randomized, controlled study. Crit Care Med. 2001;29:1860–7. doi: 10.1097/00003246-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Jaskille AD, Jeng JC, Jordan MH. Methylene blue in the treatment of vasoplegia following severe burns. J Burn Care Res. 2008;29:408–10. doi: 10.1097/BCR.0b013e31816677b5. [DOI] [PubMed] [Google Scholar]
- 13.Belletti A, Musu M, Silvetti S, Saleh O, Pasin L, Monaco F, et al. Non-adrenergic vasopressors in patients with or at risk for vasodilatory shock. A systematic review and meta-analysis of randomized trials. PLoS One. 2015;10:e0142605. doi: 10.1371/journal.pone.0142605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasin L, Umbrello M, Greco T, Zambon M, Pappalardo F, Crivellari M, et al. Methylene blue as a vasopressor: A meta-analysis of randomised trials. Crit Care Resusc. 2013;15:42–8. [PubMed] [Google Scholar]
- 15.Blacker SN, Whalen FX. Vasoplegic syndrome: Does the timing of methylene blue matter? J Anesth Clin Res. 2013;4:333. [Google Scholar]
- 16.Leyh RG, Kofidis T, Strüber M, Fischer S, Knobloch K, Wachsmann B, et al. Methylene blue: The drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass? J Thorac Cardiovasc Surg. 2003;125:1426–31. doi: 10.1016/s0022-5223(02)73284-4. [DOI] [PubMed] [Google Scholar]
- 17.Shanmugam G. Vasoplegic syndrome – The role of methylene blue. Eur J Cardiothorac Surg. 2005;28:705–10. doi: 10.1016/j.ejcts.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Koelzow H, Gedney JA, Baumann J, Snook NJ, Bellamy MC. The effect of methylene blue on the hemodynamic changes during ischemia reperfusion injury in orthotopic liver transplantation. Anesth Analg. 2002;94:824–9. doi: 10.1097/00000539-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Ginimuge PR, Jyothi SD. Methylene blue: Revisited. J Anaesthesiol Clin Pharmacol. 2010;26:517–20. [PMC free article] [PubMed] [Google Scholar]


