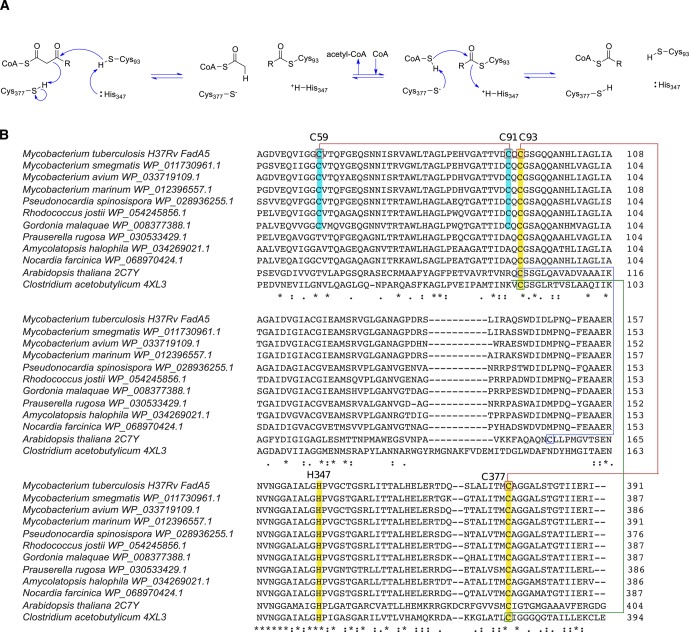
Figure 1.
Cysteine conservation in thiolases. (A) Catalytic mechanism of FadA5 thiolase. (B) Sequence alignments of thiolases from different species. The conserved catalytic residues (Cys93, His347, and Cys377) are highlighted in yellow and labeled on the basis of the amino acid numbering in FadA5. Cys 59 and Cys 91 are only present in FadA5 orthologues and are highlighted in cyan. Disulfide bonds are present in Arabidopsis thaliana (blue), Clostridium acetobutylicum (green), and Mycobacterium tuberculosis (red). (Sequence alignments were obtained using Clustal Omega. Next to the protein sequences, PDB codes, if structures are available, or NCBI Reference Sequence Project accession numbers were added.)