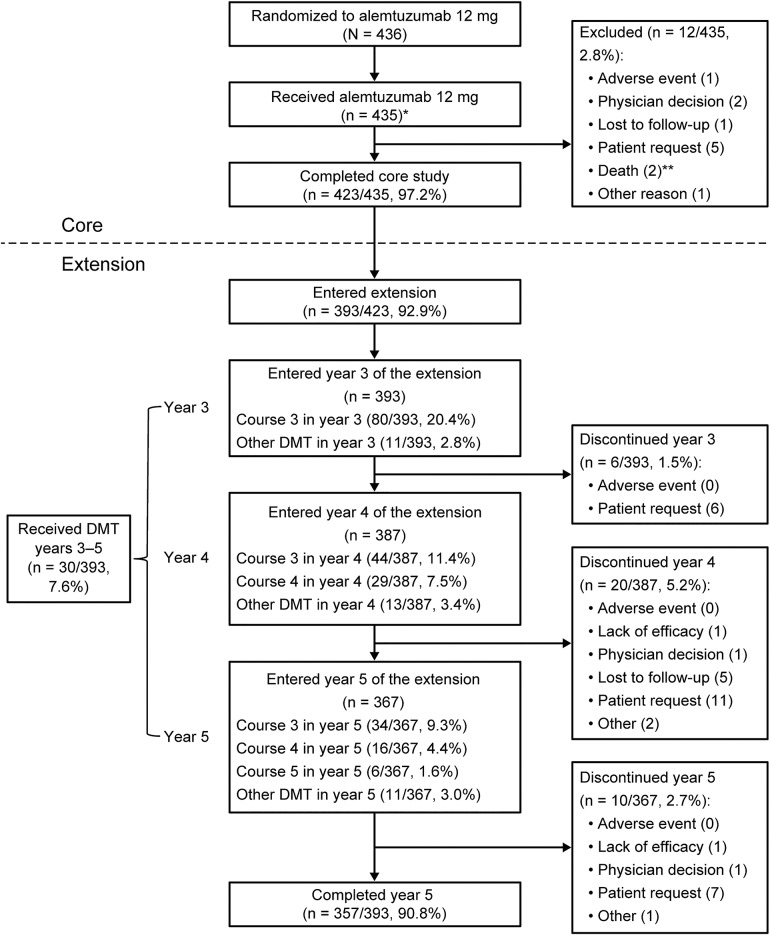
Figure 1. Patient disposition.
The disposition schematic includes participation of patients treated with alemtuzumab 12 mg in the core CARE-MS II and then enrolled in the long-term extension study. *The as-treated population (n = 435) consisted of 426 patients originally randomized to alemtuzumab 12 mg and an additional 9 patients who were randomized to alemtuzumab 24 mg but who instead received alemtuzumab 12 mg/d in the core study. **Neither death that occurred in the core study was related to treatment. CARE-MS = Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis; DMT = disease-modifying therapy.