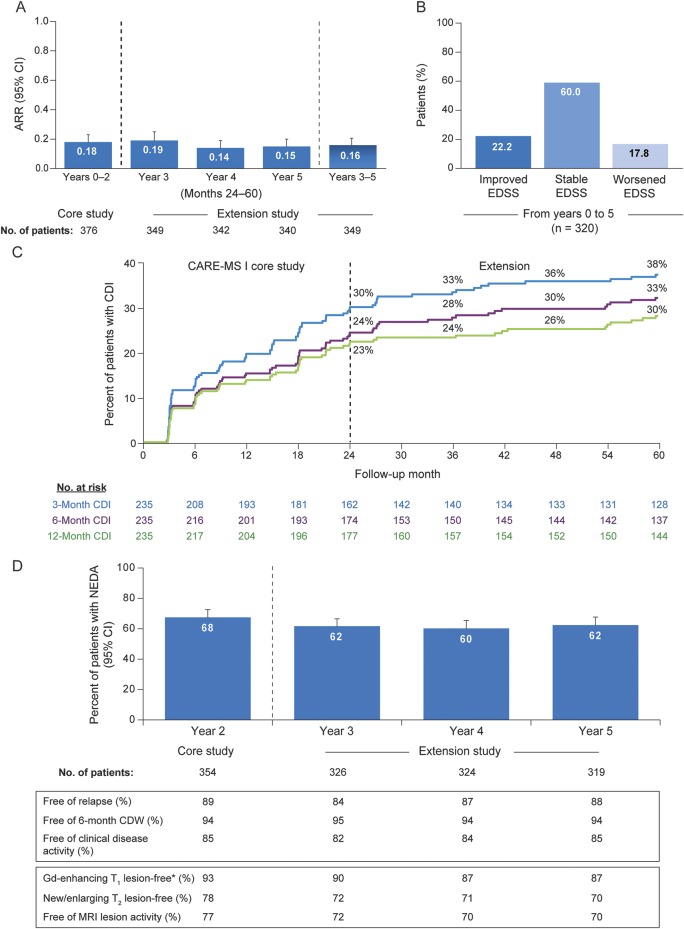
Figure 2. Clinical efficacy and disease activity outcomes over 5 years in alemtuzumab patients.
(A) ARR over 5 years in alemtuzumab patients. Results are shown for all patients who received alemtuzumab 12 mg in the core CARE-MS I study and then enrolled in the extension. A post hoc analysis revealed no statistically significant difference between ARRs in individual extension years (years 3, 4, and 5) and the ARR in years 0–2. (B) EDSS score change in alemtuzumab patients over 5 years. Proportion of patients with improved (≥1.0-point decrease), stable (≤0.5-point change), or worsened (≥1.0-point increase) EDSS scores at year 5 compared with core study baseline. EDSS score changes are shown for all patients who received alemtuzumab 12 mg in the core study and enrolled in the extension. (C) Proportion of alemtuzumab patients with 3-, 6-, or 12-month CDI over 5 years. Kaplan-Meier analysis of time to 3-, 6-, or 12-month CDI is shown for all patients who received alemtuzumab 12 mg in the core CARE-MS I study and then enrolled in the extension. (D) Proportion of alemtuzumab patients with NEDA over 5 years. Results are shown for all patients who received alemtuzumab 12 mg in the core CARE-MS I study and then enrolled in the extension. *Baseline percentage of patients Gd-enhancing lesion-free: 54%. ARR = annualized relapse rate; CARE-MS = Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis; CDI = confirmed disability improvement; CDW = confirmed disability worsening; EDSS = Expanded Disability Status Scale; Gd = gadolinium; NEDA = no evidence of disease activity.