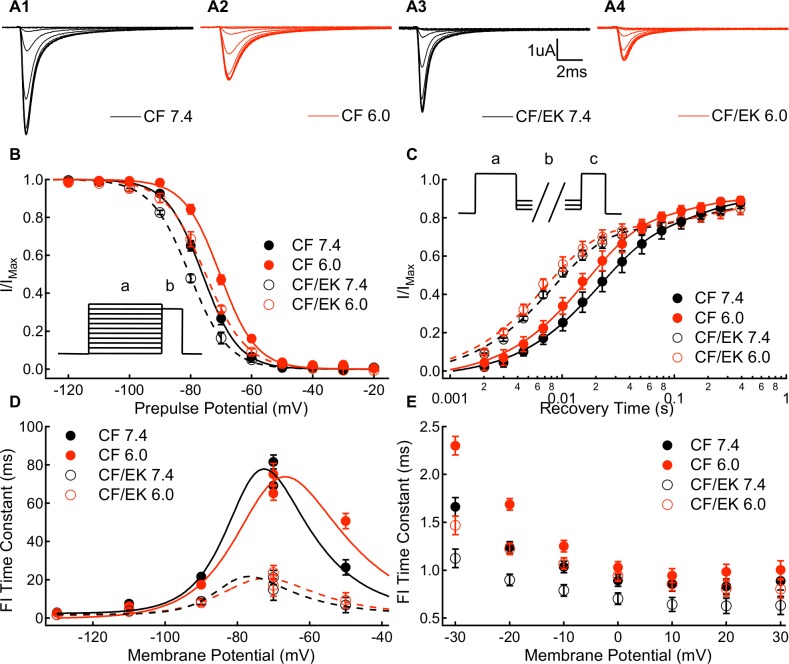
Fig 2. E1784K shifts thevoltage-dependence of fast inactivation to more hyperpolarized potentials and accelerates the rate of onset and recovery.
(A1-A4) Sample ionic currents elicited during steady-state fast inactivation recordings from C373F and C373F/E1784K NaV1.5 at pH 7.4 and pH 6.0. (B) Steady-state fast inactivation relationships for C373F and C373F/E1784K NaV1.5 at pH 7.4 and pH 6.0. The E1784K mutant shifts the midpoint of the fast inactivation voltage-dependence in the hyperpolarizing direction, whereas decreasing extracellular pH to pH 6.0 shifts the midpoint of the fast inactivation voltage-dependence to more depolarized membrane potentials. (B inset) To measure steady-state fast inactivation, cells were depolarized to -10mV (b) following a conditioning pulse to membrane potentials between -150mV and -10mV (a). (C) Recovery from fast inactivation time course at -90mV for C373F and C373F/E1784K NaV1.5 at pH 7.4 and pH 6.0. The E1784K mutant accelerates recovery from inactivation. (C inset) To measure recovery from inactivation, cells were depolarized to -10mV (c) following a conditioning pulse to 0mV (a) and a recovery pulse of variable duration to membrane potentials between -130mV and -70mV (b). (D) Time constants of fast inactivation recovery (-130mV to -70mV) and closed-state fast inactivation onset (-70mV and -50mV) plotted versus voltage for C373F and C373F/E1784K NaV1.5 at pH 7.4 and pH 6.0. The E1784K mutant accelerates fast inactivation recovery and closed-state fast inactivation onset at all potentials. (E) Time constants of open-state fast inactivation onset for C373F and C373F/E1784K NaV1.5 at pH 7.4 and pH 6.0. The E1784K mutant accelerates open-state inactivation at all membrane potentials excluding +20mV.