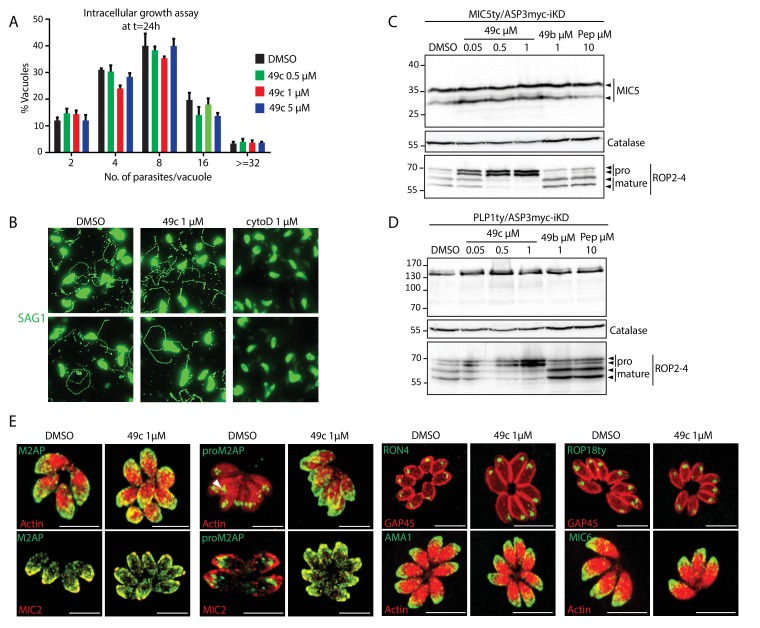
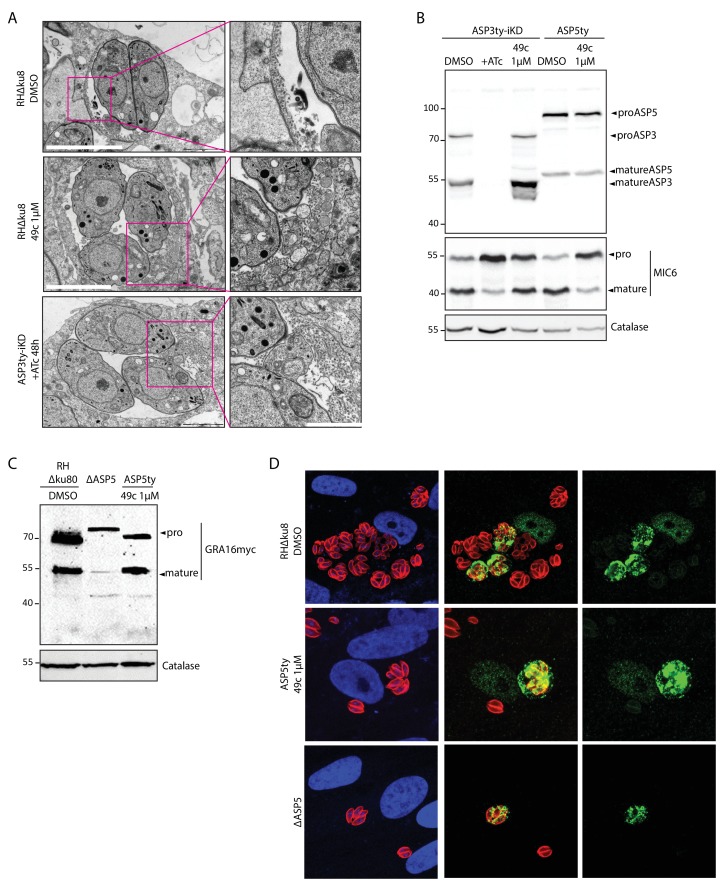
Figure 6. 49c recapitulates the phenotype of ASP3 depletion in T. gondii.
(A) Chemical structure of compounds 49b and 49c. (B) IC50 on T. gondii RH-pTub-CBG99-luciferase parasites of 49b (13.8 μM), 49c (676 nM) and Pyrimethamine (295 nM), used here as a positive control. (C) 49c treatment impaired RH parasites invasion and (D) egress. Data are presented as mean ±standard deviation (SD) from 3 independent experiments. (E, F) Parasites, treated for 36 hr with DMSO or 49c, showed normal secretion of microneme proteins (MIC2, M2AP, MIC4), but altered processing in this fraction (MIC2, M2AP, MIC4). Arrowheads show full length and secreted fragments. Catalase was used as cytosolic control and GRA1 for constitutive secretion. (G) 49c treated during 48 hr of Δku80 parasites displayed a complete absence of rhoptry content secretion as assessed by probing against the rhoptry protein ROP1. (H) Processing of RON4, ROP18 and MIC6 was affected by 48 hr of 49c treatment while DMSO treated samples were not affected. AMA1 serves a control for an unprocessed microneme and Catalase as loading control. ROP18 was Ty-tagged at endogenous locus. Pepstatin was used as a negative control. (I) Invasion was blocked by 49c when parasites were treated for 6 hr or more prior to egress, but not less than 3 hr or when extracellular. (J) Parasites were blocked in egress when treated with 49c at least 3 hr before induced egress, but not later. (K) Schematic showing the various treatments for the induced egress assay in J.