Abstract
One of the biggest challenges in treating leprosy is the control of reaction events. Patients with lepromatous leprosy may present reaction type II, or erythema nodosum leprosum, during treatment, and this reaction can remain in a recurrent form after being released from the hospital, requiring the use of thalidomide and/or prednisone for long periods of time, in turn increasing the risk of side effects. Two reports of the use of antiTNF to treat erythema nodosum leprosum were found in the literature. A good response was found after an assay with infliximab and etanercept. This study reports on a patient with lepromatous leprosy and recurrent reaction, controlled by using etanercept and a 10-month follow-up, with the interruption of thalidomide and the maintenance of prednisone at 10 mg/day.
Keywords: Leprosy, Erythema nodosum, Tumor necrosis factor-alpha
Leprosy is a chronic skin and peripheral nerve disease, with a clinical spectrum related to the immunity of the patient. Acute inflammatory episodes, called 'reactions', can occur before diagnosis, as well as during or after the end of treatment. Thirty to fifty percent of patients develop this reaction, subdivided into type I reaction, or reverse reaction, and type II reaction, or erythema nodosum leprosum (ENL).1
The type II reaction is typical of the Virchowian (lepromatous leprosy) pole. The skin presents erythematous and painful nodules throughout the body (ENL), with systemic symptoms. Light cases of the disease can be treated with analgesics and non-steroid anti-inflammatory drugs, whereas in moderate to severe cases, the treatment of choice is thalidomide and corticosteroids. However, these drugs bring about important side effects, and effective alternative schemes would be useful in many cases.1
The present case is of a 40-year-old man, who was treated for lepromatous leprosy from March 19, 2010, to April 18, 2011. He has presented repeated episodes of ENL since the original diagnosis, which have been controlled with thalidomide at a dose of 100-300 mg/day and prednisone at 20 to 40mg/day. The episodes persisted during the entire treatment period and continued even after the end of treatment. The reactions recurred as soon as the treatment with anti-rejection drugs was interrupted.
In December 2014, after having signed the Free and Informed Consent Form and having been submitted to laboratory triage, the patient began treatment with 50mg of etanercept by subcutaneous injection. Thalidomide was interrupted, and prednisone was continued at a dose of 10mg/day. Follow-up showed that the nodules presented a nearly full regression in 48 hours and that, after seven days, the disease once again recurred, requiring a new dose of etanercept (Figure 1). These intervals without reaction extended to 10 days. In September 2015, after interrupting prednisone, the patient presented a more severe reaction, which was controlled with a temporary increase of prednisone to 20mg (Figure 2). Presently, after 11 months of follow-up, the patient has presented no further reactions, performing weekly applications of 50mg of etanercept and 10mg of prednisone.
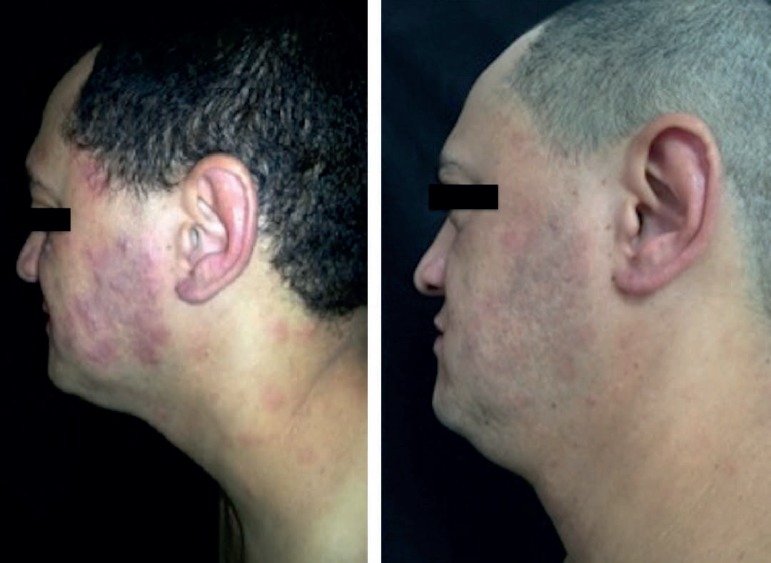
Figure 1.
Regression of the lesions 48 hours after administration of etanercept
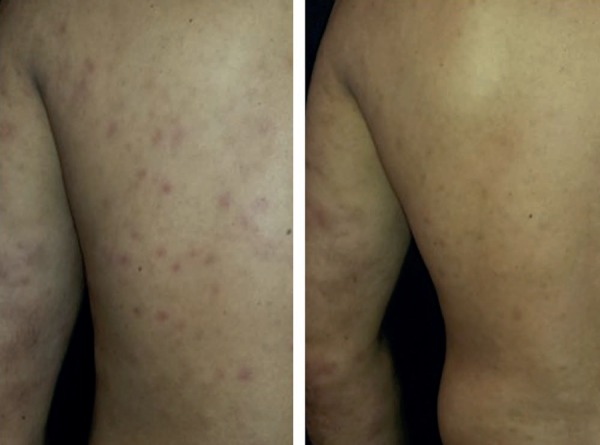
Figure 2.
Reactional exacerbation when attempting to interrupt prednisone. Control of the medical condition with a temporary increase in the dose to 20mg/day
The type II leprosy reaction, mediated by immunocomplexes, increases the pro-inflammatory cytokines (tumor necrosis factor alpha - TNF-alpha - and interferon gamma - IFN-gamma), inducing cytotoxic activity and bacillary destruction, followed by the intensification of cytokine secretion, which activates the immune response.1
In 1991, Sarno et al. showed a high concentration of IL-1 and THF-alpha in leprosy reactions.2 Brenan et al. also verified that, with the use of thalidomide, 90% of the patients with ENL had diminished the release of TNF-alpha.3
Thalidomide contains an anti-inflammatory and immunomodulator activity due to the degradation of the TNF-alpha's messenger RNA, the diminishing of CD4 lymphocytes, and the inhibition of interleukins (IL) 12, 4, and 5, as well as the inhibition of INF-gamma.1
Stimulated by the thalidomide action in the control of the production of TNF-alpha, some authors have shown good results of the drug and of pentoxifylline in the therapeutic handling of diseases, such as rheumatoid arthritis, psoriasis, and Crohn's disease, in which this cytokine participates.4-6 In 1995, Talhari et al. reported that pentoxifylline interferes in the TNF-alfa and IL-1 levels, which act against leprosy reactions, and should therefore be considered an alternative drug in the handling of ENL.7
The introduction of specific drugs in the TNF-alpha antagonism had a heavy impact on the treatment of the aforementioned diseases. In a literature review, two reports were found in the off-label use of these drugs against the type II reaction of leprosy. In the two cases, ENL presented a good response when infliximab and etanercept were used.8,9
In 2006, Faber et al. used infliximab, a chimerical anti-monoclonal antibody, in a patient diagnosed with dimorphic lepromatous leprosy, treated with polychemotherapy, together with ENL that is non-responsive to prednisone, pentoxifylline, and thalidomide. Infliximab (5 mg/kg) was administered, while the anti-rejection drugs that were being used were suspended. After 24 hours, the ENL symptoms had been significantly reduced. After two administrations of infliximab during weeks 2 and 6, and with a follow-up of one year, no further episodes of ENL were described.8
In the second report, Ramien et al. used etanercept, a humanized fusion protein analogous to the TNF receptor and that is linked to soluble TNF, on a male patient diagnosed with recurrent and persistent ENL with a six year evolution, previously treated with thalidomide and corticosteroids, in turn blocking leprosy's ligation to the trans-membrane-cell receptors. In a dose of 50 mg/week, a good response was observed after six weeks, at which time the corticosteroids were interrupted. Thalidomide was suspended after six months and used again after one year of therapy due to a reactional episode, which lasted an additional three months. Treatment with etanercept was continued for two years, and the patient has now gone 2 ½ years with no further symptoms, according to his report.9
In this case report, though there was a good response to etanercept, the reaction is currently only controlled with the administration of the drug in weekly doses. Thalidomide was interrupted, but the attempt to suspend prednisone was followed by a significant reactional outbreak.
Another important point to be considered, in Brazil, is the high risk of the reactivation of latent tuberculosis when using anti-TNF drugs (greater with infliximab when compared to etanercept). The removal of thalidomide and the maintenance of prednisone in low doses shows that the biological agents can be useful in patients with type II leprosy, which is difficult to control, and are capable of reducing the need for thalidomide and prednisone, thus sparing patients of the side effects from both drugs. Therefore, studies conducted with a greater number of patients and longer follow-ups, in an attempt to evaluate and define the risks and benefits of these new drugs, are warranted.
Footnotes
Conflict of interest: none.
Work conducted at the Professor Rubem David Azulay Dermatology Institute, Santa Casa de Misericórdia do Rio de Janeiro (DPRDA-SCMRJ) - Rio de Janeiro, (RJ), Brazil.
Financial support: none.
REFERENCES
- 1.Talhari S, Penna GO, Gonçalves HS, Oliveira MLW. Hanseníase. 5.ed. Manaus: Dilivros; 2015. [Google Scholar]
- 2.Sarno EN, Grau GE, Vieira LM, Nery JA. Serum levels of tumour necrosis factor-alpha and interleukin-1β during leprosy reactional states. Clin Exp Immunol. 1991;84:103–108. [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PF, Chatterjee D, Brennan PJ, Rea TH, Modlin RL. Tumor necrosis factor production in patients with leprosy. Infect Immun. 1992;60:1441–1446. doi: 10.1128/iai.60.4.1441-1446.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huizinga TW, Dijkmans BA, van der Velde EA, van de Pouw Kraan TC, Verweij CL, Breedveld FC. An open study of pentoxifylline and thalidomide as adjuvant therapy in the treatment of rheumatoid arthritis. Ann Rheum Dis. 1996;55:833–836. doi: 10.1136/ard.55.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigoni ACM, Carneiro SCS. Estudo aberto com pentoxifilina em pacientes com psoríase. An Bras Dermatol. 2001;76:39–49. [Google Scholar]
- 6.Bauditz J, Wedel S, Lochs H. Thalidomide reduces tumour necrosis factor α and interleukin 12 production in patients with chronic active Crohn's disease. Gut. 2002;50:196–200. doi: 10.1136/gut.50.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talhari S, Orsi AT, Talhari AC, Souza FH, Ferreira LC. Pentoxifiline may be useful in the treatment of type 2 leprosy reaction. Lepr Rev. 1995;66:261–263. [PubMed] [Google Scholar]
- 8.Faber WR, Jensema AJ, Goldschmidt WF. Treatment of recurrent erythema nodosum leprosum with infliximab. N Engl J Med. 2006;355:739–739. doi: 10.1056/NEJMc052955. [DOI] [PubMed] [Google Scholar]
- 9.Ramien ML, Wong A, Keystone JS. Severe refractory erythema nodosum successfully treated with the tumor necrosis factor inhibitor etanercept. Clin Infect Dis. 2011;52:e133–e135. doi: 10.1093/cid/ciq213. [DOI] [PubMed] [Google Scholar]