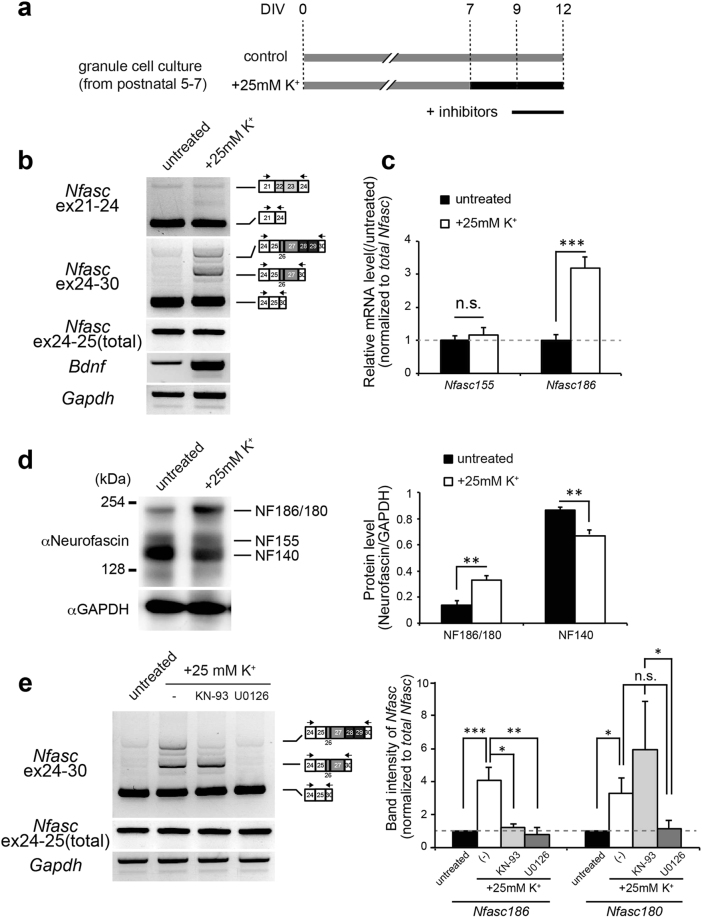
Figure 5.
Depolarization-dependent alternative splicing of neuronal Nfasc in cerebellar GCs. (a) Schematic diagram of the in vitro pharmacological experiment. Cerebellar GCs were maintained in normal K+ media (5 mM, control; grey bar) or high-K+ media (final 30 mM, intervention; black bar) from 7 days in culture (DIV) through 12 DIV. The pharmacological agents were applied for 3 days before harvest (under black line). (b) Representative images of switch in the splicing of Nfasc caused by high K+-induced depolarization. Depolarization strongly induced inclusion of ex26-29 in cerebellar GCs. Brain-derived neurotrophic factor (Bdnf) expression was monitored to confirm immediate-early gene induction. (c) RT-qPCR analysis of the depolarized GC culture. Ex27-28, included in Nfasc186, was increased as a result of high-K+ stimulation. (n = 6 cultures) Values for the untreated culture were set to 1.0. (d) Western blot analysis of total cell lysates from untreated control and depolarized cultures with anti-neurofascin antibody. Shown is a shift in neuronal NF isoform in cerebellar GC culture due to high K+-induced depolarization. Intensities of protein bands corresponding to NF186 and NF140 were normalized to that of GAPDH. (n = 3 cultures). (e) Pharmacological experiments in depolarized GC cultures. KN-93 (CaMK selective inhibitor, 10 µM), and U0126 (ERK inhibitor, 5 µM) were used to block Ca2+-dependent signalling. Values for untreated GCs were arbitrarily defined as 1.0. (n > 4 cultures). Nfasc186: F (3,24) = 8.374 (P < 0.0001); Nfasc180: F (3,36) = 8.423 (P < 0.0001), one-way ANOVA. Differences were compared to the untreated group using the post-hoc test (Dunnett’s test), after one-way ANOVA.