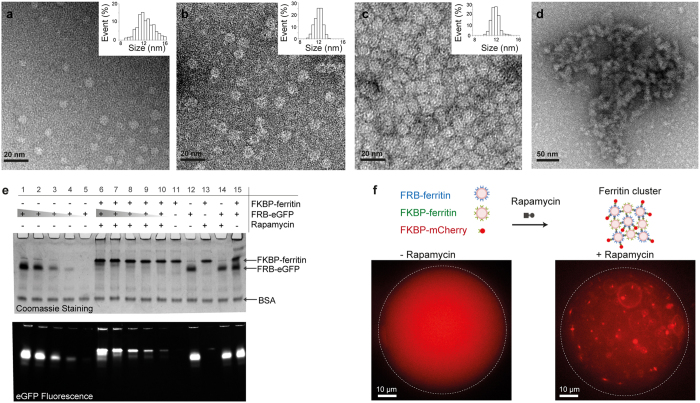
Figure 2.
Characterisation of ferritin assemblies and biofunctionalisation. (a–d) Transmission electron microscopy of negatively stained (a) FRB-ferritins, (b) FKBP-ferritins, (c) native ferritins, and (d) ferritin clusters (inserts: size distribution histograms). (e) Acrylamide gel electrophoresis of FKBP-ferritins, FRB-eGFP proteins, and eGFP-ferritin complexes. From lane 11 to 15, 10 µM of FRB-eGFP and 10 µM of FKBP-ferritin migrate freely, in absence (lanes 11, 12 and 15) or presence (lanes 13 and 14) of 20 µM of Rapamycin. On lanes 1 to 5, the monomeric profile of 15–10–5–2.5–1 µM of FRB-eGFP is assessed in presence of Rapamycin. On lanes 6 to 10, according to the initial stoichiometry, the migration FRB-eGFP is retarded in presence of Rapamycin and 10 µM of FKBP-ferritin. In each well, BSA was added as loading control. (f) Schematic of ferritin cluster formation (top). Fluorescence images of a water droplet containing FRB-ferritins, FKBP-ferritins, and FKBP-mCherry (left). The ferritin clusters are formed in presence of Rapamycin (right).