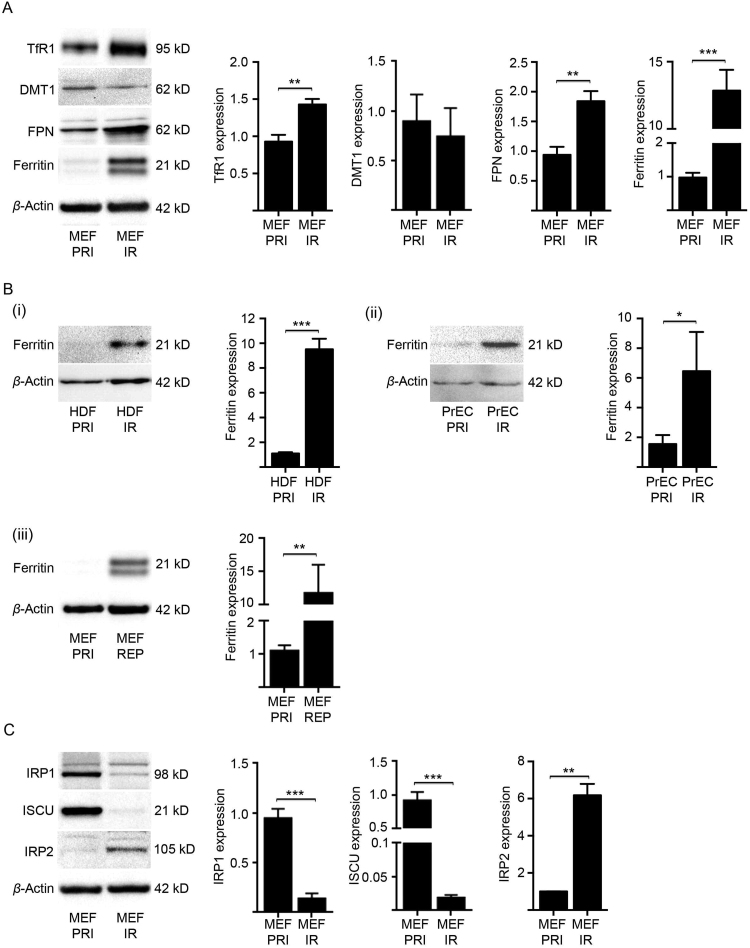
Fig. 3.
Altered iron homeostatic mechanisms drive senescent cells to acquire intracellular iron. (A) Expression of key iron homeostasis proteins was analysed in senescent MEFs (IR) at 21 days post-irradiation (10 Gy) by western blot and densitometry. The levels of transferrin receptor 1 (TfR1) [main iron (Fe3+) importer], divalent metal transporter 1 (DMT1) (cytosolic iron importer), ferroportin (iron exporter) and ferritin (intracellular iron storage) were measured in comparison to primary (PRI) MEFs. β-actin was detected as a loading control. (B) Western blot analyses and densitometry confirm elevated expression of ferritin in (i) senescent human diploid fibroblasts (HDF IR) and in (ii) senescent human prostate epithelial cells (PrEC IR), both at 21 days post-irradiation (10 Gy). Furthermore, elevated ferritin expression was confirmed in (iii) replicative senescent MEFs (P7) cultured for 21 days. β-actin was detected as loading controls. (C) Expression of key regulatory proteins of iron homeostasis was analysed in senescent MEFs (IR) at day 21 post-irradiation (10 Gy) by western blot and densitometry. The levels of iron regulatory protein 1 (IRP1) and iron-sulfur cluster assembly enzyme (ISCU) were found significantly lower than those in primary (PRI) MEFs, while IRP2 was significantly elevated. β-actin was detected as a loading control. Statistical analysis was performed by student-t-test: significant (*p < 0.05, **p < 0.01, ***p < 0.001). Data represented as mean ± SD (n = 3).