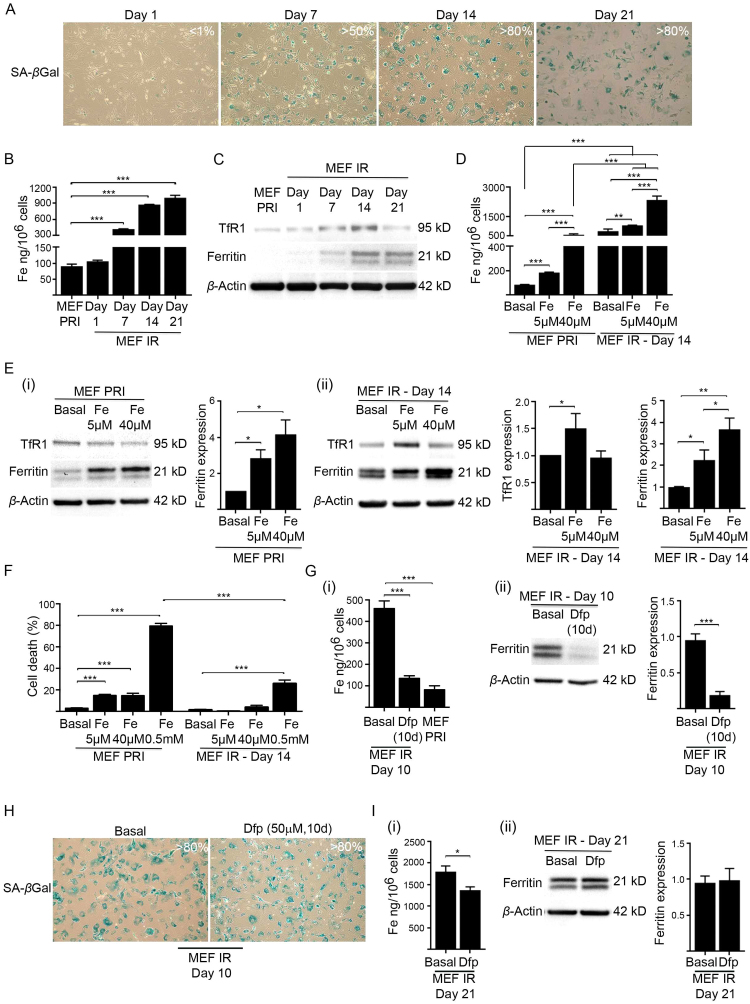
Fig. 4.
Cellular senescence precedes iron accumulation and provides resistance to iron induced toxicity. (A) Percentage of senescent MEFs (MEF IR) post-irradiation (10 Gy) over the culturing time points indicated (day 1, 7, 14 and 21), as determined by SA-βgal activity (blue staining). Images were taken at 100× magnification. (B) ICP-MS analyses demonstrated cumulative intracellular iron accumulation in irradiated (10 Gy) senescent MEFs (MEF IR) over the culturing time points indicated (day 1, 7, 14 and 21). Note that iron accumulation somewhat plateaued after day 14, but was maintained. Baseline intracellular iron level in primary MEFs (MEF PRI) is also shown. (C) Expression of iron uptake and storage proteins was analysed in senescent MEFs (IR) post-irradiation (10 Gy) over the culturing time points indicated (day 1, 7, 14 and 21) by western blot. The levels of transferrin receptor 1 (TfR1) [main iron (Fe3+) importer] and ferritin (intracellular iron storage) were determined in comparison to primary (PRI) MEFs. β-actin was detected as a loading control. (D) Capacity of senescent MEFs to accumulate iron is dependent on milieu iron concentration. Primary (PRI) MEFs or irradiated (10 Gy) senescent MEFs (IR) (cultured for 14 days) were cultured for 24 h in basal medium, or media supplemented with either 5 µM or 40 µM iron (FAC). ICP-MS analyses revealed a dose-dependent increase in accumulated iron in primary and senescent MEFs (IR) following iron treatment. (E) Senescent MEFs are geared to sequester milieu iron. (i) Western blot analyses demonstrated that TfR1 was downregulated and ferritin upregulated (fold change indicated by densitometry) when primary MEFs (MEF PRI) were cultured for 24 h in media supplemented with either 5 µM or 40 µM iron (FAC). (ii) Western blot analyses and densitometry demonstrated that TfR1 and ferritin are further upregulated when senescent cells (14 days post-irradiation) were cultured for 24 h in media supplemented with either 5 µM or 40 µM iron (FAC). β-actin was detected as loading controls. (F) Senescent MEFs possess enhanced resistance to elevated iron. The viability of iron treated primary and senescent MEFs was determined by propidium iodide and flow cytometry. Note that an extremely high concentration of iron (500 µM) was used as a positive control. (G) Chelation reduced intracellular iron and ferritin accumulation in irradiated MEFs. Primary MEFs were treated with the cell-permeable iron chelator deferiprone (Dfp) (50 µM) while irradiated (10 Gy) to become senescent and then subsequently for 10 days in culture. Primary MEFs irradiated and cultured in basal medium served as a control. (i) ICP-MS analyses demonstrated that sustained Dfp treatment prevented iron accumulation in irradiated MEFs. Baseline intracellular iron level in primary MEFs (MEF PRI) is also shown. (ii) Western blot analyses and densitometry demonstrated that sustained Dfp treatment prevented ferritin accumulation in irradiated MEFs. β-actin was detected as a loading control. (H) Percentage of senescent MEFs following sustained Dfp treatment. Primary MEFs were treated with Dfp (50 µM) while irradiated (10 Gy) to become senescent and then subsequently for 10 days in culture. Primary MEFs irradiated and cultured in basal medium served as a control. The majority (> 80%) of treated MEFs become senescent, staining positive for SA-βgal activity (blue staining).(I) Stored iron in senescent MEFs once accumulated is highly resistant to chelation. Senescent MEFs (21 days post-irradiation) treated with Dfp (50 µM) for 24 h showed (i) only a marginal decrease in intracellular iron as determined by ICP-MS. (ii) Western blot analyses and densitometry showed a negligible effect of Dfp treatment on ferritin level of senescent MEFs. β-actin was detected as a loading control. Statistical analysis was performed by student-t-test: significant (*p < 0.05, **p < 0.01, ***p < 0.001). Data represented as mean ± SD (n = 3).