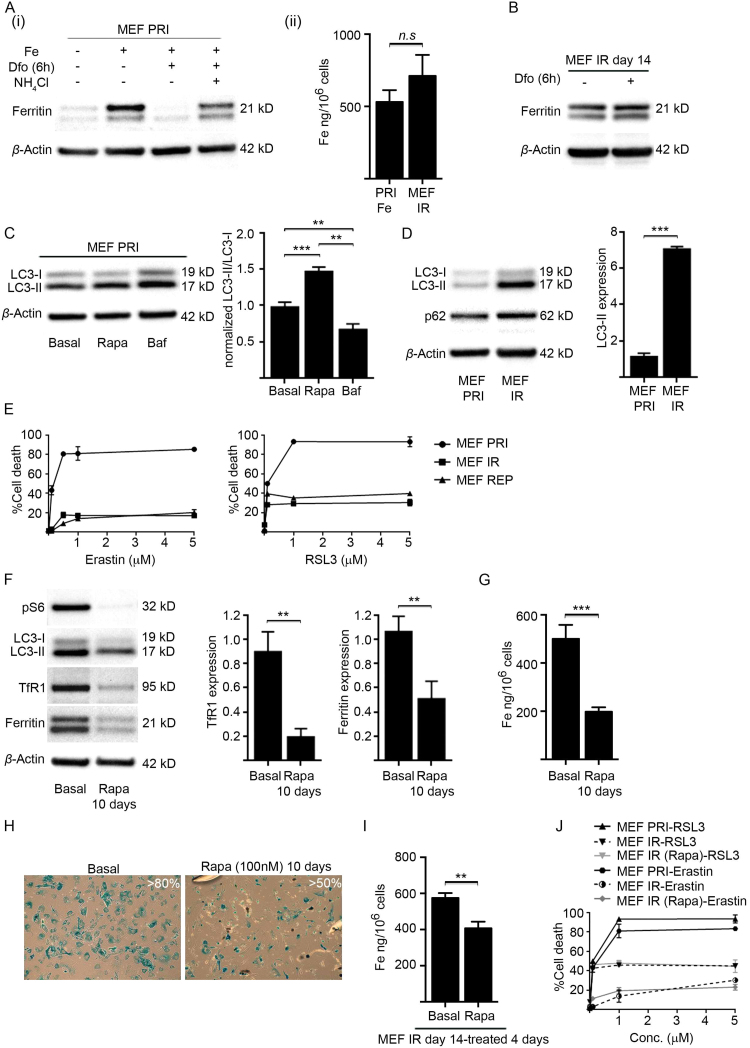
Fig. 5.
Iron accumulation in senescent MEFs is associated with impaired ferritinophagy. (A) Ferritin degradation in primary MEFs (MEF PRI) was inhibited by lysosomal acidification. (i) Primary MEFs (MEF PRI) were treated with 40 µM iron (FAC) for 24 h to increase ferritin expression. Ferritin degradation was stimulated with the iron chelator deferoxamine (Dfo) (50 µM) and occurred substantially with 6 h of treatment, as determined by western blot analyses (ii) ICP-MS analyses confirmed that intracellular iron levels increased in primary MEFs following treatment with 40 µM iron (FAC) for 24 h (PRI Fe), subsequently becoming comparable to iron levels observed in senescent MEFs (MEF IR) (14 days post-irradiation).(B) Ferritin degradation in senescent MEFs (MEF IR) is impaired. Western blot analysis showing no change to ferritin in senescent MEFs (MEF IR) (14 days post-irradiation) treated with Dfo (50 µM) for 6 h. β-actin was detected as a loading control.(C) Autophagy in primary MEFs with functional lysosomes can be monitored by the LC3-II: LC3-I ratio. The ratio of LC3-II: LC3-I was determined by western blot analyses and densitometry and increased in primary MEFs (MEF PRI) treated with the autophagy activator rapamycin (100 nM) for 16 h. The ratio of LC3-II: LC3-I was decreased in primary MEFs (MEF PRI) treated with the autophagy inhibitor bafilomycin A1 (100 nM) for 16 h. β-actin was detected as a loading control. (D) Senescent MEFs (10 days post-irradiation) displayed a large build-up of LC3-II and elevated levels of p62, indicating impaired lysosomal function, as determined by western blot analyses and densitometry. The levels of LC3-II protein and p62 in primary MEFs (MEF PRI) is shown for comparison. β-actin was detected as a loading control. (E) Senescent MEFs are highly resistant to ferroptosis. The viability of primary and senescent MEFs treated for 24 h with varying concentrations of erastin (0.1–5 μM) and RSL3 (0.1–5 μM) was determined by propidium iodide and flow cytometry. (F) Preservation of autophagy averted the accumulation of iron-regulatory proteins in irradiated MEFs. Primary MEFs were treated with rapamycin (100 nM) while irradiated (10 Gy) to become senescent and then subsequently for 10 days in culture. Western blot analyses demonstrated that sustained rapamycin treatment significantly reduced expression of phosphorylated ribosomal protein S6 (pS6). LC3-II levels were likewise significantly reduced indicating preservation of lysosomal function and permissible LC3-II degradation. Rapamycin treatment averted the iron accumulation phenotype of senescent MEFs, preventing the accumulation of TfR1 and ferritin (densitometry shown). β-actin was detected as a loading control. (G) Preservation of autophagy averted the iron accumulation in irradiated MEFs. Primary MEFs were treated with rapamycin (100 nM) while irradiated (10 Gy) to become senescent and then subsequently for 10 days in culture. Primary MEFs irradiated and cultured in basal medium served as a control. ICP-MS analyses demonstrated that sustained rapamycin treatment averted intracellular iron accumulation in irradiated MEFs. (H) Percentage of senescent MEFs following sustained rapamycin treatment. Primary MEFs were treated with rapamycin (100 nM) while irradiated (10 Gy) to become senescent and then subsequently for 10 days in culture. Primary MEFs irradiated and cultured in basal medium served as a control. Approximately 50% of treated MEFs became senescent, staining positive for SA-βgal activity as established by blue staining. Images were taken at 100× magnification. (I) Autophagy activation reduced stored iron in established senescent MEFs. ICP-MS analyses demonstrated that senescent MEFs (MEF IR) (10 days post-irradiation) treated for 4 days with rapamycin (100 nM) showed a significant reduction in intracellular iron when compared to untreated senescent MEFs (MEF IR). (J) Preservation of autophagy was insufficient to sensitize irradiated MEFs to ferroptosis. The viability of primary, senescent, and rapamycin treated irradiated MEFs (MEF IR + Rapa) (as described previously in Fig. 5G), treated for 24 h with varying concentrations of erastin and RSL3 (0.1–5 μM) was determined by propidium iodide and flow cytometry. Statistical analysis was performed by student-t-test: significant (*p < 0.05, **p < 0.01, ***p < 0.001). Data represented as mean ± SD (n = 3).