FIG 1 .
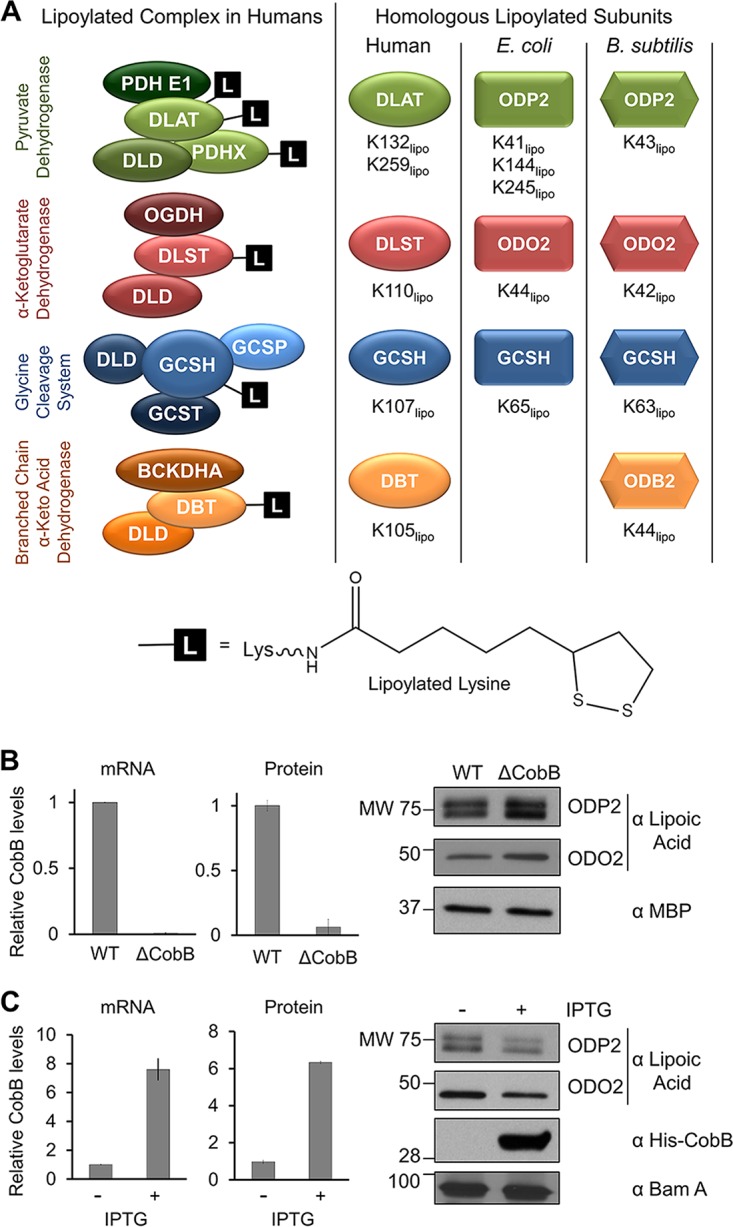
CobB levels are inversely correlated with protein lipoylation levels in E. coli. (A) Lipoylated protein complexes are conserved across humans, E. coli, and B. subtilis; L depicts lipoylation. Homologous complex subunits and modified lysine residues (Klipo) are indicated. (B) Assessment of CobB mRNA levels via qPCR (left), of CobB protein levels via MS (middle), and of lipoyl levels via Western blotting (right) in the ΔcobB strain relative to WT. MBP was used as a loading control. SRM-MS/MS quantification (middle) showed relative levels of a representative unmodified CobB peptide, KYYGPASQVVPE. The detected signal in the ΔcobB strain was at the level of noise in the MS analysis (i.e., undetected CobB). (C) Assessment of CobB mRNA levels, CobB protein levels, and lipoyl levels in the +IPTG group relative to the uninduced strain, as shown in panel B. The SRM-MS/MS detected signal in the uninduced cells (middle) was derived from combined MS noise and leaky plasmid expression. IPTG induction of His-CobB was seen using anti-His antibody; BamA was used as a loading control. Error bars represent standard deviations.
