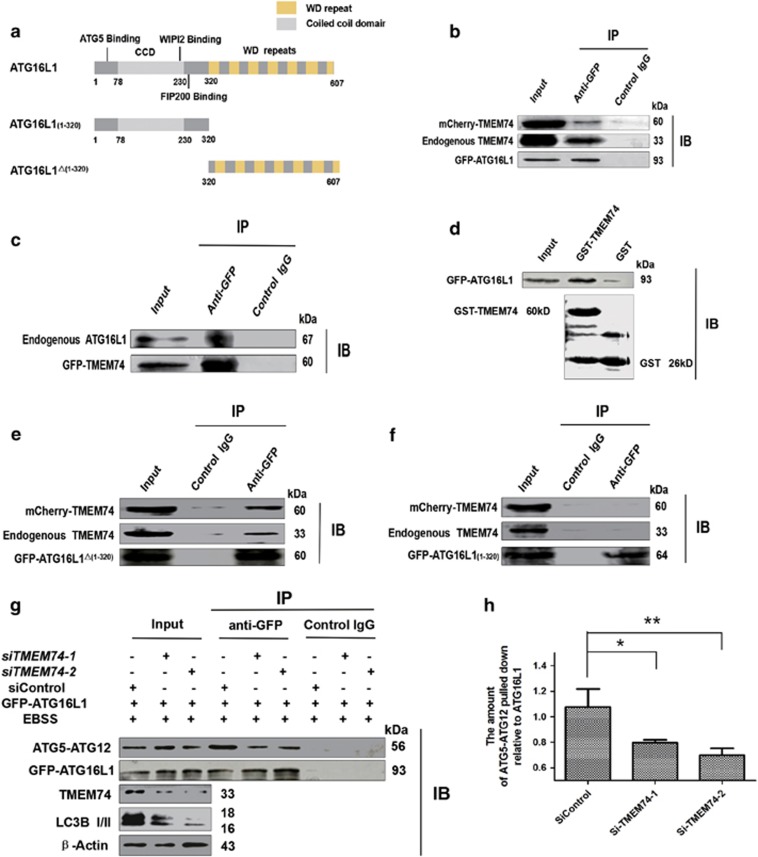
Figure 2.
TMEM74 associates with ATG16L1 via its C-terminal and influences the interaction between ATG5 and ATG16L1. (a) Schematic representations of WT ATG16L1 and its mutants: ATG16L1(1–320), and ATG16L1△(1–320). (b) HeLa cells were co-transfected with GFP-ATG16L1 and mCherry-TMEM74 for 24 h, Total cell extracts were subjected to IP using either an anti-GFP or an isotype control IgG, TMEM74 was detected in the washed beads using anti-TMEM74 IgG by western blotting. (c) HeLa cells were co-transfected with GFP-TMEM74 and mCherry-ATG16L1 for 24 h. Total cell extracts were subjected to IP using either an anti-GFP or an isotype control IgG, ATG16L1 was detected in the washed beads using an anti-ATG16L1 IgG by western blotting. (d) GST and GST-TMEM74 fusion protein immobilized on glutainione-sepharose beads were incubated with HeLa cell lysates containing GFP-ATG16L1, GFP-ATG16L1 was detected in the washed beads by western blotting. (e,f) HeLa cells were co-transfected with mCherry-TMEM74 and GFP-ATG16L1(1–320), or GFP-ATG16L1△(1–320) respectively for 24 h. Total cell extracts were subjected to IP using an anti-GFP or an isotype control IgG, as indicated. TMEM74 were detected in the washed beads by western blotting. (g,h) HeLa cells were firstly treated by siTMEM74-1,siTMEM74-2 or siControl for 24 h, then transfected with GFP-ATG16L1 for 24 h, meanwhile treated with EBSS for at least 8 h. Total cell extracts were subjected to IP using an anti-GFP or a non-specific control IgG, ATG5-ATG12 complex pulled down was detected in the immunoprecipitates using anti-ATG5 by western blotting. Quantification of ATG5-ATG12 pulled down relative to GFP-ATG16L1 was shown as column. Data are means±S.D. of three experiments. *P<0.05, **P<0.01