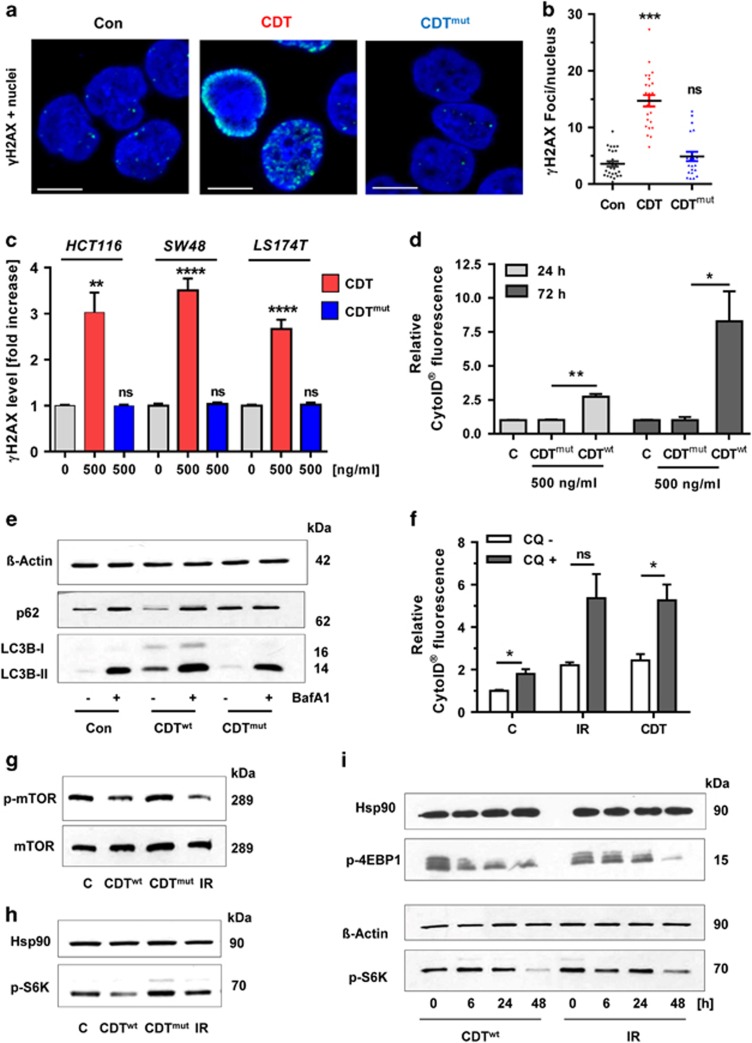
Figure 2.
DSB formation is required for autophagy induction and mTOR repression. (a) Induction of γ-H2AX foci by wild-type CDT and mutant CDT (CDTmut) lacking DNase activity. HCT116 cells were treated with CDT or CDTmut for 24 h (500 ng/ml each), stained with a γ-H2AX antibody and subjected to confocal microscopy. Representative images are shown. γ-H2AX is depicted in green and nuclei are blue. Scale bar represents 10 μm. (b) Quantitative evaluation of γ-H2AX foci in HCT116 cells as shown in panel (a). The number of γ-H2AX foci per nucleus were determined by the ImageJ software and evaluated with GraphPad Prism 5.0 (>50 cells per experiment; n=3); NS: not significant. ***P<0.001, versus control. (c) ICW analysis of γ-H2AX formation in different CRC cell lines treated with wild-type or mutant CDT. Cells were exposed to CDT or its DNase I-defective mutant (CDTmut) as described above. After 24 h, cells were processed for ICW analysis and γ-H2AX level was determined as compared with untreated control cells (n=5). NS: not significant. **P<0.01, ****P<0.0001. (d) Analysis of autophagosome formation in HCT116 cells upon treatment with DNase I-deficient CDT. Cells were incubated with CDT or CDTmut (500 ng/ml each) for 24 or 72 h. Induction of autophagosomes was revealed by the CytoID Autophagy Detection Kit and flow cytometry. n=3; **P<0.01, *P<0.05. Bars in light gray: 24 h; bars in dark gray: 72 h. (e) Monitoring of autophagic flux. HCT116 cells were incubated with CDT or CDTmut for 21 h, then supplemented or not with the autophagy inhibitor Baf A1 (10 nM) and incubated for another 3 h. After 24 h, cells were harvested and analyzed by SDS-PAGE followed by western blotting detection of LC3B and p62. β-Actin served as loading control. (f) HCT116 cells were treated with CDT or exposed to IR. After 30 h, the autophagy inhibitor CQ (10 μM) was added and cells were further incubated for 18 h. Cells were harvested after 48 h and autophagy induction was measured using the CytoID Autophagy Detection Kit (n=3). NS: not significant. *P<0.05. (g) Impact of DSB induction on mTOR signaling. HCT116 cells were treated with CDT, CDTmut or IR and harvested after 48 h. Samples were then separated by SDS-PAGE and analyzed by western blotting detection of mTOR and phospho-mTOR. (h) Detection of the mTOR substrate phospho-p70-S6K in cells treated as described above for 24 h. Hsp90 served as loading control. (i) Time-dependent repression of mTOR signaling by CDT and IR. HCT116 cells were exposed to CDT or IR and incubated for up to 48 h. Samples were then analyzed as described above followed by detection of the mTOR substrate phospho-p70 S6K and phospho-4E-BP1