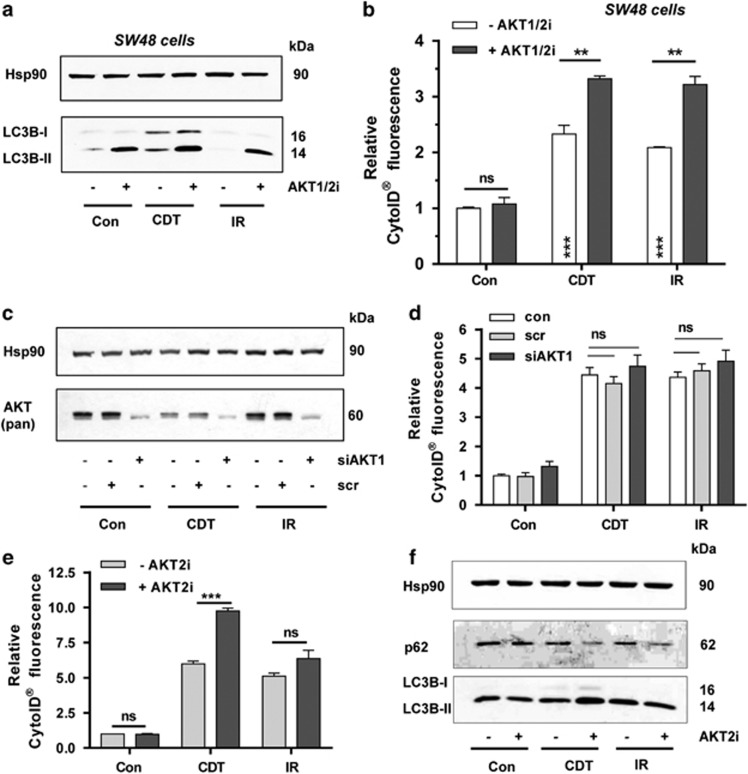
Figure 6.
Isoform-dependent suppression of DSB-induced autophagy by AKT. (a) Impact of AKT on LC3B accumulation in SW48 cells. The cells were treated with CDT (500 ng/ml) or irradiated (10 Gy) in the absence or presence of an AKT1- and AKT2-specific inhibitor (AKT1/2i; 0.5 μM). Cells were harvested after 48 h and subjected to western blotting analysis as indicated. Hsp90 served as loading control. (b) Induction of autophagosomes in SW48 cells in the presence of absence of AKT signaling. Cells were challenged as described above, processed for CytoID staining and then analyzed by flow cytometry. (n⩾3); ***P<0.001, **P<0.01, NS: not significant. (c) Transient siRNA-mediated knockdown of AKT1. HCT116 cells were transfected with AKT1 siRNA or scrambled siRNA. After 24 h, cells were exposed to CDT (500 ng/ml) or IR (10 Gy) and incubated for additional 48 h followed by western blotting analysis using a pan-AKT antibody. Hsp90 served as loading control. (d) AKT1 knockdown and DSB-induced autophagosomes. Cells were treated as described in panel (c) and autophagosome formation was monitored by CytoID staining and flow cytometry. (n=3). NS: not significant. (e) Pharmacological inhibition of AKT2 and formation of autophagic vesicles following DSB generation. HCT116 cells were treated with CDT (500 ng/ml) or exposed to IR (10 Gy) and incubated for 48 h in the absence or presence of an AKT2-specific inhibitor (AKT2i; 1 μM). Autophagosome formation was measured using the CytoID Staining Kit and analyzed by flow cytometry. (n⩾3); ***P<0.001; NS: not significant. (f) AKT2 inhibition and DSB-induced LC3B accumulation and p62 degradation. Cells were treated as described in panel (e) and subjected to western blotting analysis for LC3B and p62. Hsp90 was detected as loading control