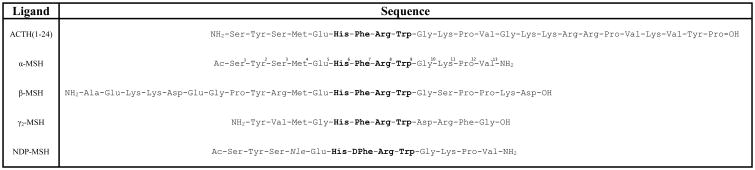
Figure 1.
Amino acid sequence of the endogenous POMC melanocortin agonists and NDP-MSH synthetic analog. The numbering of α-MSH is indicated. The tetrapeptide sequence His-Phe-Arg-Trp, common to all naturally occurring MCR agonists, is indicated in bold. The more potent NDP-MSH ligand is modified from α-MSH by replacing Met4 with Nle and inverting the stereochemistry at the Phe7 position.