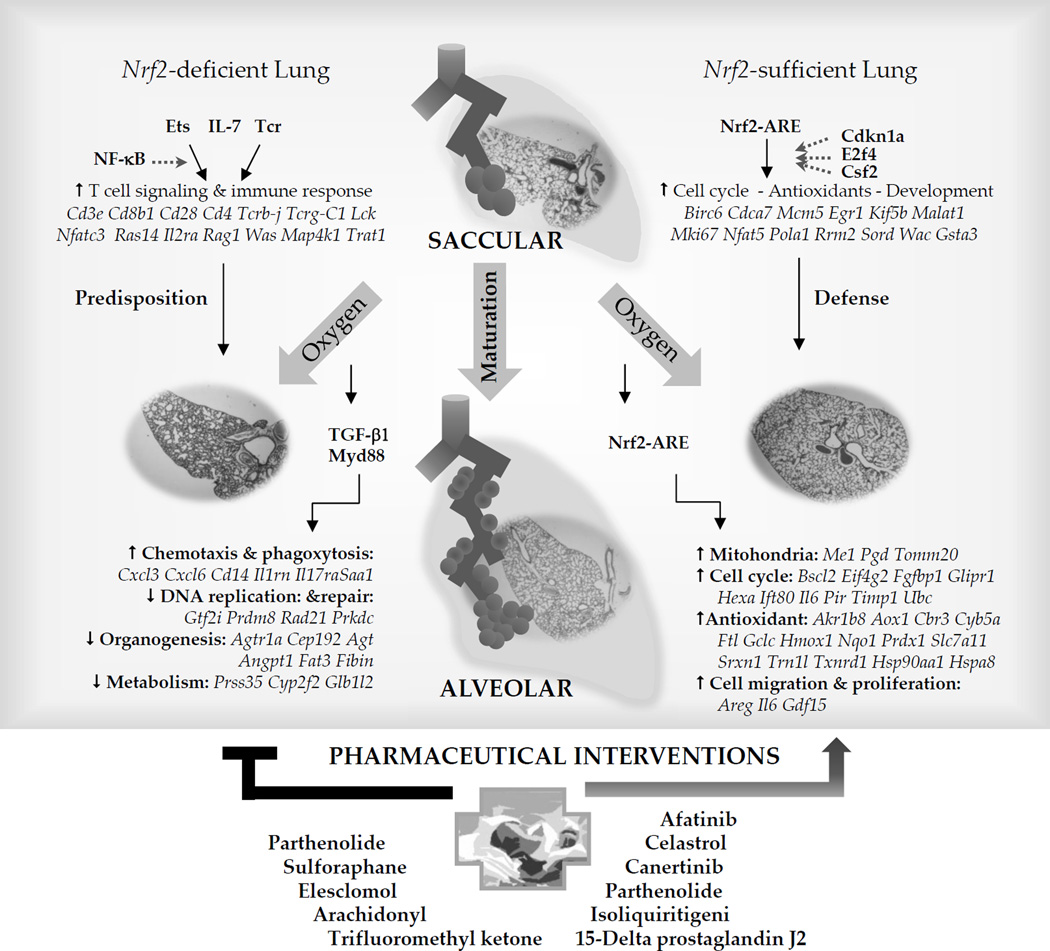
Figure 2. Proposed Nrf2-mediated regulatory mechanisms of saccular-to-alveolar transition and bronchopulmonary dysplasia (BPD)-like pathogenesis and potential therapeutic intervention.
Comparative microarray analysis of Nrf2+/+ and Nrf2−/− neonates followed by bioinformatics suggested downstream Nrf2 target genes bearing functional AREs in the lung undergoing saccular-to-alveolar transition and BPD-like pathogenesis. Molecules such as cell cycle dependent kinase 1a (Cdkn1a), E2F transcription factor 4 (F2f4), or colony stimulating factor 2 (Csf2) may participate in the Nrf2-ARE pathway during normal saccular-to-alveolar transition. Hyperoxia exposure to the premature lung at saccular phase with simple, poorly septated saccules causes lung injury and interrupts normal alveolar formation. Nrf2-mediated protection is predicted to be through transcriptional activation of antioxidant, cell cycle and DNA repair, and mitochondria-related metabolism. Nrf2 deficiency in saccular phase lung suppressed these cellular processes and aberrantly activated upstream molecules including transforming growth factor beta 1 (TGF-β1) and myeloid differentiation primary response gene 88 (Myd88) may drive inflammatory cell infiltration and attenuate DNA repair and cell cycle, metabolism, and morphogenesis signaling. Augmented transcriptome of T lymphocyte signaling and related acquired immunity during the saccular-to-alveolar transition Nrf2−/− neonate lung may predispose the BPD-like phenotypes. Ets family transcription factor, interleukin 7 (IL-7), or T cell receptor (Tcr) in association with nuclear factor kappa-light-chain enhancer of activated B cells (NF-kB) may play roles in the upstream of this process. Drugs or chemicals acting on redox balance (e.g., sulforaphane, Celastrol), anti-inflammation (e.g., 15-delta prostaglandin J2, Isoliquiritigenin, Pathenolide, Celastrol), or anti-cancer (e.g., Elesclomol, Pathenolide, Canertinib, Afatinib) mechanisms are suggested as pharmaceutical interventions for BPD pathogenesis.