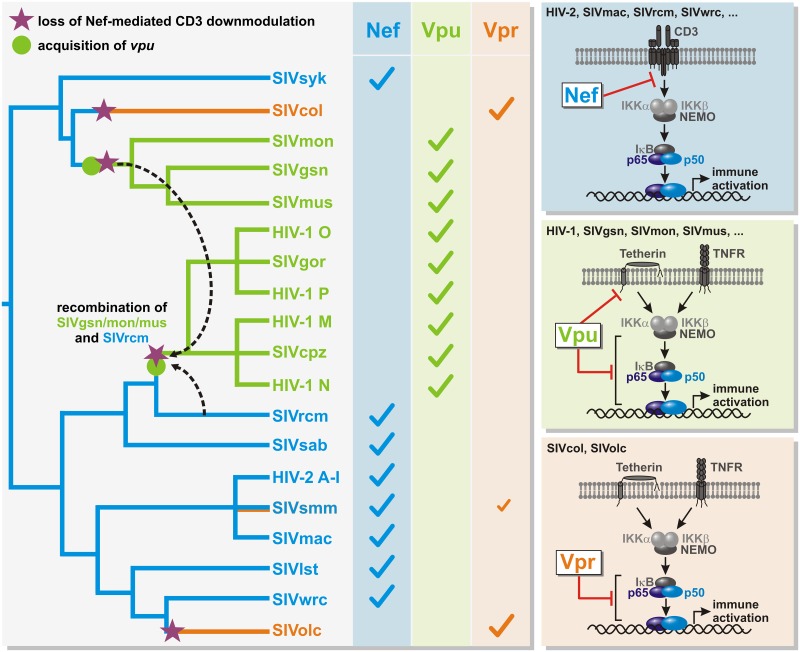
Fig 8. Vpu-, Vpr- and Nef-mediated inhibition of immune activation.
The left panel illustrates the emergence of Vpu-, Vpr- and Nef-mediated suppression of immune activation during primate lentiviral evolution: Most primate lentiviruses use their Nef proteins to prevent T cell activation via downmodulation of CD3 (blue). HIV-1, SIVcpz/gor as well as SIVgsn/mon/mus encode a vpu gene and evolved Vpu-mediated inhibition of NF-κB activation (green). Nef-mediated CD3 downmodulation was lost in these viruses (violet star). In SIVcol and SIVolc, the loss of CD3 downmodulation by Nef is associated with the evolution of Vpr-mediated suppression of NF-κB signaling (orange). The tree is a schematic representation of primate lentiviral evolution and based on previous phylogenetic analyses [12]; branch lengths do not correlate with phylogenetic relationships. While Nef directly targets the CD3 receptor to prevent downstream NF-κB signaling, Vpu and SIVcol/SIVolc Vpr target the canonical NF-κB signaling cascade further downstream, independently of the receptor (right panels). Some Vpu proteins additionally counteract the host restriction factor Tetherin, which also acts as an immune sensor.