Figure 2.
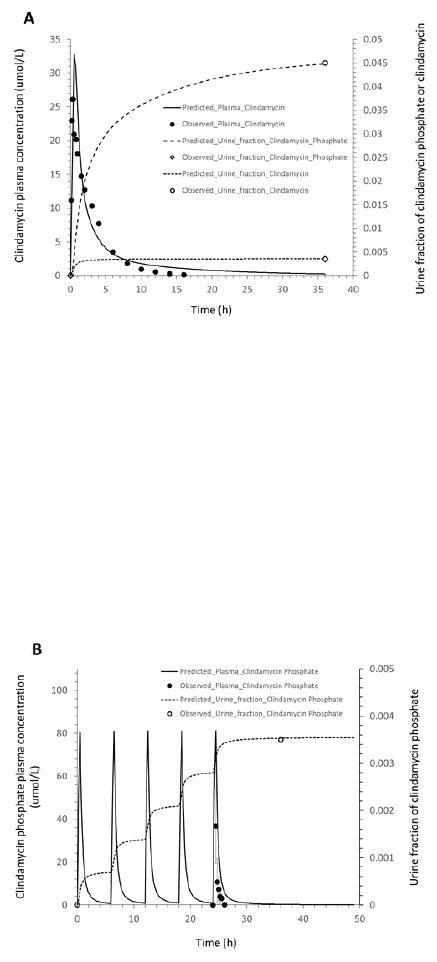
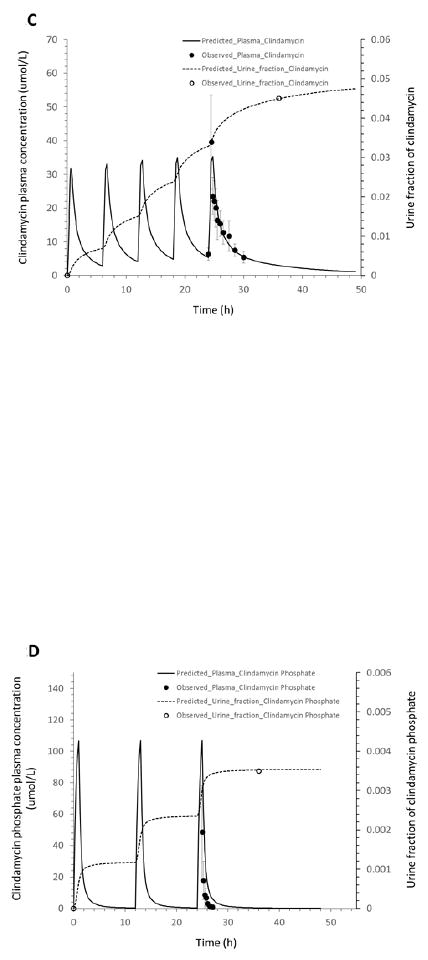

Mean observed (dots) and simulated (lines) plasma concentrations and fractions of the drug excreted unchanged in urine in healthy adults: (A): clindamycin concentration in plasma, and clindamycin and clindamycin phosphate fractions in urine after a 30-min intravenous infusion of 600 mg clindamycin phosphate; (B): clindamycin phosphate concentration in plasma and fraction in urine after IV administration of 600 mg clindamycin phosphate every 6 hours; (C): clindamycin concentration in plasma and fraction in urine after IV administration of 600 mg clindamycin phosphate every 6 hours; (D): clindamycin phosphate concentration in plasma and fraction in urine after IV administration of 1200 mg clindamycin phosphate every 12 hours; (E): clindamycin concentration in plasma and fraction in urine after IV administration of 1200 mg clindamycin phosphate every 12 hours.
