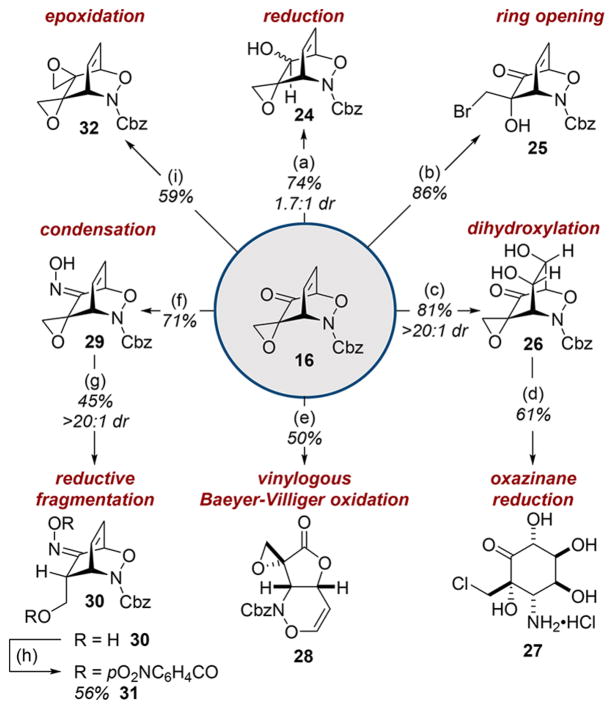
Scheme 2. Chemoselective Reactions of Heterocycloadducts*.
*Conditions: aNaBH4 (0.33 equiv), THF, 0 °C; bBr2 (2.0 equiv), Me2S (2.5 equiv), CH2Cl2, 0 °C to rt; cOsO4 (1 mol %), NMO (6.0 equiv), THF/acetone/H2O (5:5:1), 0 °C to rt; di. Pd/C (10 wt %), AcOH (5.0 equiv), MeOH, rt, ii. HCl in 1,4-dioxane (4 N, 25.0 equiv), rt; emCPBA (1.5 equiv), NaHCO3 (2.0 equiv) CH2Cl2, rt; fHONH2· HCl (1.5 equiv), NaOAc (2.0 equiv), THF, rt; gNaBH4 (2.0 equiv), anhydrous MeOH, 0 °C; hp-O2NC6H4COCl (2.2 equiv), TEA (3 equiv), DMAP (10 mol %), anhydrous THF, rt; iNaH (60 wt %, 1.1 equiv), Me3SO·I (1.1 equiv), anhydrous THF, 0 °C.