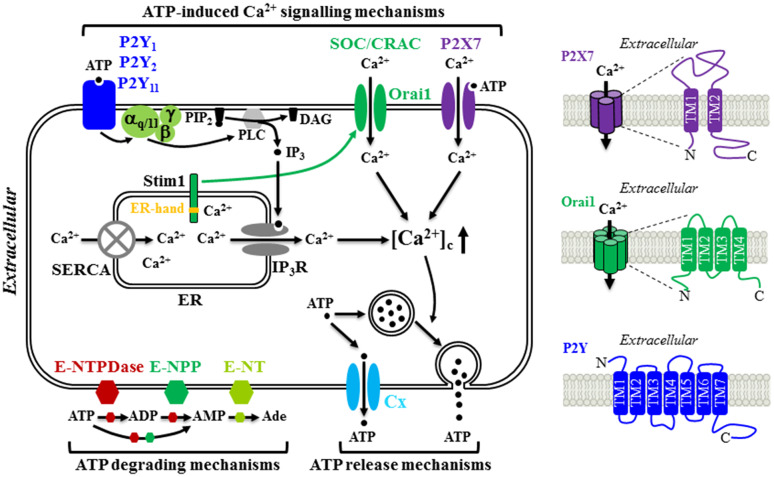
Fig. 1.
Schematic diagram illustrating the molecular mechanisms for ATP release and hydrolysis, and ATP-induced Ca2+ signalling in MSC. MSC release ATP constitutively through connexin (Cx) hemi-channels and in response to mechanical stimuli via vesicular exocytosis. Extracellular ATP are hydrolyzed to ADP and AMP by ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases) and ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP) and further to adenosine (Ade) by ecto-5′-nucelotidase (E-NT). Once outside the cell, ATP acts as an autocrine or paracrine signalling molecule by elevating the cytosolic Ca2+ concentrations ([Ca2+]c) through three molecular mechanisms. ATP can induce activation of the P2X7 receptor ion channel allowing extracellular Ca2+ influx. Alternatively, ATP can activate the P2Y1, P2Y2 and/or P2Y11 receptor, leading to sequential activation of Gα,q/11, phospholipase C (PLC), conversion of membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol triphosphate (IP3) and diacylglycerol (DAG), activation of the receptor for IP3 (IP3R) and Ca2+ release from the endoplasmic reticulum (ER). Depletion of the ER Ca2+, upon activation of the Gα,q/11-PLC-IP3R signalling pathway or blockage of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) induces extracellular Ca2+ entry via the store-operated Ca2+ (SOC) or Ca2+ release activated Ca2+ (CRAC) channel. Stim1 acts as the ER Ca2+ sensor via the EF-hand motifs located in the ER lumen (denoted by yellow strip) to monitor the ER Ca2+ level. Reduction in the ER Ca2+ level induces conformal changes in Stim1, leading to its translocation to and trapping at the ER-plasma membrane junction, where it interacts with the Orai1 protein to open the Ca2+-permeating channel. Further details and references are described in the text. The structural features of the P2X7 receptor, Orai1 channel and P2Y receptor are illustrated on the right