Figure 3.
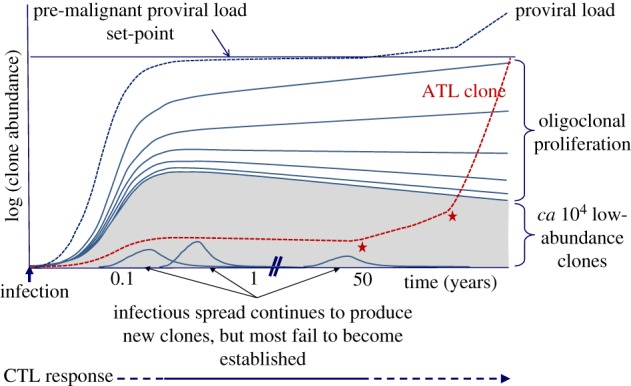
Schematic depiction of HTLV-1 clonality over the course of infection. Each line on the figure represents the growth of one HTLV-1+ T-cell clone; the grey shaded area represents a large number of lower-abundance clones. The red dashed line shows the growth trajectory of a clone that undergoes malignant transformation after 50 years; the red asterisks denote the acquisition of tumour driver mutations; in ATL these are frequent in certain signalling pathways: T-cell receptor; NFκB; CCR4; p53; and Notch-1 [84–87]. The pre-malignant clone is shown to originate in early infection; this is likely [31,88], but not necessary. During the first few weeks of infection, before the emergence of an effective cytotoxic T-lymphocyte response, the virus spreads rapidly by cell-to-cell contact through the virological synapse; the number of clones of infected T lymphocytes typically rises to 104 to 105 when the proviral load set-point is reached, after approximately one to two months [23,89]. In the chronic phase of infection, CTLs restrict this infectious mode of spread [44], and the proviral load is maintained by continued proliferation of existing clones. In this phase, there is a quasi-equilibrium between viral propagation and the host immune response; while the proviral load remains approximately constant, the abundant clones grow in abundance and the low-abundance clones shrink, leading to a progressive rise in the oligoclonality index [37]. The abundant clones appear to last for the lifetime of the host [37]. During chronic infection the abundant, persistently activated anti-Tax CTL response demonstrates that Tax expression is frequent in vivo [45]. Since Tax expression is normally undetectable in fresh PBMCs, we infer that Tax expression is intermittent in vivo. Virus-specific CTLs may persist during active ATL; it remains to be tested whether boosting the CTL response can be used as an adjunct to therapy. Constant cell division leads to the accumulation of replicative mutations, which increase the probability of malignant transformation [90]. ATL usually arises after 4–6 decades of infection, and so is more frequent in individuals infected during childhood. The risk of ATL may also be correlated with the proviral load, which in turn is correlated with the number of HTLV-1-infected T-cell clones, not with the degree of oligoclonality [37].
