Abstract
A growing body of literature demonstrates that racial group membership can influence neural responses, e.g. when individuals perceive or interact with persons of another race. However, little attention has been paid to social class, a factor that interacts with racial inequalities in American society. We extend previous literature on race-related neural activity by focusing on how the human brain responds to racial out-groups cast in positively valued social class positions vs less valued ones. We predicted that the ventromedial prefrontal cortex (vmPFC) and the amygdala would have functionally dissociable roles, with the vmPFC playing a more significant role within socially valued in-groups (i.e. the middle-class) and the amygdala having a more crucial role for socially ambivalent and threatening categories (i.e. upper and lower class). We tested these predictions with two complementary studies: (i) a neuropsychological experiment with patients with the vmPFC or amygdala lesions, contrasted with brain damaged and normal comparison participants, and (ii) a functional magnetic resonance imaging experiment with 15 healthy adults. Our findings suggest that two distinct mechanisms underlie class-based racial evaluations, one engaging the vmPFC for positively identified in-group class and another recruiting the amygdala for the class groups that are marginalized or perceived as potential threats.
Keywords: race, social class, fMRI, lesion, emotions
Introduction
While overt forms of racial bias have been declining in USA (Schuman et al., 1997; Madon et al., 2001; Hunt, 2007), covert prejudice is still prevalent (Pager and Shepherd, 2008; Bonilla-Silva, 2010). This prevalence has contributed to a shift of attention to implicit forms of racial bias (Greenwald and Banaji, 1995; Nosek et al., 2007) as well as a growing interest in uncovering the brain mechanisms involved in racial evaluations (Hart et al., 2000; Richeson et al., 2003, 2008; Lieberman et al., 2005). Racial group membership influences neural responses in the brain (Gutsell and Inzlicht, 2012; Wang et al., 2015), but racial evaluations are not made in a vacuum. Cues such as eye gaze direction, ethnic group membership, culturally based self-construals and social-cognitive goals (e.g. categorization vs individuation) modulate race related brain activation (Wheeler and Fiske, 2005; Chiao et al., 2008; Richeson et al., 2008; Trawalter et al., 2008; Wang et al., 2015). The study reported here extends this literature by focusing on how perceived social class membership modulates race-related neural responses.
The United States is a highly stratified society with steep levels of class inequality (Western and Wright, 1994; Wright, 1997; Weeden et al., 2007). Social class is an important base for the construction of self and identities (Urciuoli, 1993; Hout, 2008) as well as group distinctions and racial disadvantages via residential and educational segregation, and interpersonal relations like homophily in marriage and friendship networks (Schneider and Buckley, 2002; Horvat et al., 2003; Hochschild and Scovronick, 2004; Schwartz and Mare, 2005). Social class positions are intrinsically linked with symbolic aspects of human social interaction anchored in moral evaluation systems (Lamont, 1992; Sayer, 2005). People separate their valued in-group class members from out-group members by ascribing the latter with lower moral standards and character (Lamont, 1992, 2000). Moral emotions are linked to feelings about in-groups and out-groups, with the former acting as an intuitive standard for moral value while out-groups triggering moral apathy to perceived violations of important taken-for-granted societal notions of propriety (Nucci and Turiel, 2000; Wainryb, 2006). While we perceive in-group members more positively and feel more empathy and sympathy, we socially (and morally) exclude out-group members, sometimes treating them as non-human beings (Haslam, 2006; Harris and Fiske, 2007; Fiske, 2009).
Sociological research linking class and racial inequalities observes that while a growing body of African Americans have transitioned into the middle class—and now faced limited overt discrimination—a substantial portion of African Americans living in the inner cities have become increasingly marginalized, constituting the urban ‘underclass’ (Wilson, 1978, 1987, 2009). However, the ways that social class cues modulate neural responses to different racial groups have not been extensively or properly explored. The most common stimuli used in neurological studies are facial pictures of Black and White individuals without any distinguishing context. Yet, human interaction relies fundamentally on context, especially valued (or not) social class markers, to make the sorts of judgments previously studied in isolation. We address this gap by investigating how the human brain responds to pictures of Blacks and Whites embedded in social contexts depicting the socially desirable middle-class vs the lower-class and the upper-class.
Neurological evidence suggests that the vmPFC is a pivotal brain structure in moral emotional evaluations and in-group/out-group processes (Damasio, 1994; Greene et al., 2001; Moll et al., 2002; Koenigs et al., 2007; Prehn et al., 2008; Adolphs, 2009; FeldmanHall et al., 2012; Sevinc and Spreng, 2014). There is increased medial prefrontal cortex activation when making judgments about physically or politically similar others (Mitchell et al., 2006), close others (e.g. friends) (Krienen et al., 2010), distributing resources to maximize in-group members’ profit (Volz et al., 2009) and reduced activation when dehumanizing stigmatized others (Harris and Fiske, 2006, 2007). The amygdala, on the other hand, has a key role in processing social signals of emotion, particularly fear (Adolphs et al., 1998, 1999; LeDoux, 2000; Phan et al., 2002; Dalgleish, 2004). While the vmPFC and the amygdala are reciprocally connected, and are both involved in moral and social appraisals (Damasio et al., 1996; Price, 1999; Berridge and Kringelbach, 2008), we suggest that they might be playing functionally dissociable roles in moral emotional processing of racial stimuli. Neuroimaging studies of race demonstrated greater amygdala activation in response to out-group racial stimuli (Hart et al., 2000; Phelps et al., 2000; Cunningham et al., 2004; Lieberman et al., 2005; Ronquillo et al., 2007). Moreover, previous research indicates that the amygdala is involved in emotional vigilance, especially in relation to ambiguous external stimuli that require more attention (Whalen, 1998; Davis and Whalen, 2001; Hamann et al., 2002). Therefore, we suggest that the amygdala’s role in racial bias is potentially more specific in terms of responses toward those in lower-class and upper-class (out-group) categories—because of the greater attention that will be spent to detect whether or not people in these groups pose a threat.
This background leads to specific predictions: The vmPFC and the amygdala will have functionally dissociable roles in racial evaluations, with the vmPFC playing a more significant role in racial differences within the middle-class and the amygdala having a more crucial role for the upper and lower classes. To test these predictions, we utilized the following: (i) a neuropsychological experiment with patients who have damage to the vmPFC or amygdala, contrasted with brain damaged and normal comparison participants, and (ii) a functional magnetic resonance imaging (fMRI) experiment with 15 healthy adults. In both experiments, participants viewed realistic photographs of Blacks and Whites in lower class, middle-class and upper-class conditions as well as pictures of pleasant and unpleasant non-human stimuli (as foil categories) and rated how they felt when viewing them on eight emotions: envy, pity, pride and disgust, anger, sadness, happiness and fear. We were particularly interested in emotional responses to these pictures because previous research suggests that emotions are not only vital mechanisms for moral judgment and action (Moll et al., 2005; Haidt, 2007, 2008) but also are stronger predictors of racial discrimination than cognitive schemata such as stereotypes (Leyens et al., 2003; Talaska et al., 2008).
Experiment 1: neuropsychological study
Participants
Seventeen neurological patients who incurred focal brain damage as adults (after age 18 years) were selected from the Iowa Patient Registry. Subjects included five patients with focal bilateral vmPFC lesions (see Figure 1 for lesion overlaps), five patients with unilateral amygdala lesions (three left and two right, see Figure 1 for lesion overlap) as well as seven brain-damaged comparison (BDC) subjects. One BDC subject who could not finish all the study procedures in the designated time period was excluded. Fifteen age-, race- and education-matched, neurologically normal comparison (NC) subjects were recruited via flyers and recruitment ads posted locally. Overall, patients did not have major defects in cognitive functioning or general intelligence, and the subgroups were similar in demographic characteristics, explicit racial evaluations and the neuropsychological scales reported here (see the online Supplementary for detailed inclusion/exclusion criteria).
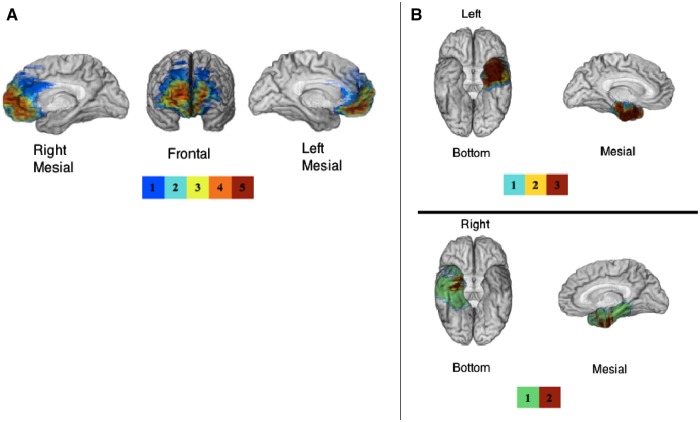
Fig. 1.
(A) Mesial and frontal views of the overlap map of lesions for the five vmPFC patients. The area of maximal overlap lies in the vmPFC. (B) Bottom and left hemisphere mesial views for of the overlap map of lesions for the three left amygdala patients and bottom and right hemisphere mesial views of the overlap map of lesions for the two right amygdala patients are presented. The highest overlap in the vmPFC group includes medial frontopolar areas and the highest overlap in the left amygdala group includes the temporal pole and perirhinal areas. For both Panel A and B, the color bar indicates the number of overlapping lesions at each voxel.
The neurological patients were all at least 30 years old and had education levels of at least 10th grade; recruited normal comparisons met these same criteria. None of the NC subjects reported to be from the highest or the lowest socio-economic strata (bottom 10th percentile= <$12 000; top 10 percentile= >$140 000).
Materials
Picture set . A total of 200 pictures (25 per factor) were used to assess subjects’ emotional responses (see Table 1), including White and Black people in three different socio-economic positions as well as pictures of non-human stimuli (e.g. objects, animals). The pictures were chosen from the International Affective Picture System (IAPS) (Lang et al., 1995) and the World Wide Web. The picture content across racial categories within each SES group (e.g. Black middle SES vs White middle SES) are matched in terms of qualitative (e.g. scenery, pose, posture) as well as quantitative aspects (number of people, visual complexity, eye-gaze) (see online Supplementary for examples, Table A1 for comparison).
Table 1.
Experimental conditions
| Lower class | Middle class | Upper-class | Objects |
|---|---|---|---|
| Black | Black | Black | Pleasant |
| White | White | White | Unpleasant |
| e.g. homeless people, people with worn-out clothing | e.g. people barbecuing, having a picnic in casual clothing | e.g. people wearing upscale clothing and jewelry, people in front of a sports car or a luxury yacht | e.g. spider, snarling dog, birthday cake, flowers |
Note: There are 25 pictures per condition (e.g. 25 pictures of Black middle-class people) in Experiment 1 (the neuropsychological study) and 24 pictures per condition in Experiment 2—the functional MRI study (in order to be able to equally distribute pictures across experimental blocks).
Procedure
Participants were told that the purpose of the study was to investigate how the brain gives emotional responses to pictures of people vs objects and non-human animals in order to reduce social desirability and awareness effects. Each picture appeared on the screen for 2 s; eight emotion labels, happy, pride, sad, pity, angry, disgust, envy and fear, appeared sequentially; participants were asked to indicate whether they felt that emotion by pressing ‘YES’ or ‘NO’. For affirmative emotions, participants were directed to a new window to indicate the extent they were feeling that emotion ranging from ‘1’ (very low) to ‘5’ (extreme). Pictures were randomized for each subject and emotion labels were randomized for each picture. A green fixation cross on a black background was presented in between each picture. This picture rating procedure took ∼1 h, after which, participants completed other scales on paper and were debriefed.
Analytical approach
Initial investigations with a principal components factor analysis suggested a two-factor solution with happiness, pride and envy loading on one factor and the rest of the emotions loading on a second factor (see online Supplementary Table A2). Therefore, we averaged happiness, pride and envy into one desirability-related emotions variable (Cronbach’s Alpha = 0.63) and created a second distressful emotions variable by averaging the sadness, pity, disgust, anger and fear (Cronbach’s Alpha = 0.82). We have analyzed the desirability-related and distressful emotion scores separately by fitting multilevel mixed-effects regression models. Preliminary investigations revealed that there were no significant differences between the BDC and normal groups on their reports of either of the emotions. To increase power and ease of interpretation, BDC and normal groups were entered as a combined category into final analyses. Models included subjects as random intercepts, with fixed effects including race of the stimuli, social class of the stimuli, lesion type, and the interactions between these variables. For significant, interaction effects, we have conducted further investigations with follow-up contrasts.
Results
Demographic characteristics
Demographic and clinical characteristics and attitude scale scores are displayed in Table 2. Patients had mostly intact cognitive functioning and general intelligence (see online Supplementary Table A3). Bonferroni comparisons reveal groups were not significantly different from each other on age, Full Scale IQ from the Wechsler Adult Intelligence Scale-III, depression as measured by the Beck Depression Inventory, PANAS negative affect scale, and the explicit racial evaluation scales (stereotype assessment, racial contact and symbolic racism). The amygdala group had significantly less education than the BDC (P = 0.04) (the vmPFC and BDC did not differ). Additionally, the vmPFC group had a significantly longer chronicity time than the BDC (P = 0.028) and a significantly lower positive affect score than the amygdala group (P = 0.025). Pearson chi-squared and Fischer’s exact tests show that the groups were not significantly different from each other on categorical variables including sex, handedness and social class.
Table 2.
Demographic and clinical characteristics, and attitude scales
| vmPFC | Amygdala | BDC | NC | Chi2/F | Pr/P value | |
|---|---|---|---|---|---|---|
| Sex | 2M, 3F | 3F, 2M | 4M, 2F | 7M, 8F | 1.10 | 0.78 |
| Age | 66 (9.6) | 55.2 (16.3) | 64 (8.8) | 63 (9.9) | 0.96 | 0.43 |
| Education | 14 (1.5) | 13 (1.1) | 16.5 (3.2) | 14.7 (1.9) | 3.18 | 0.04 |
| Social class | 3M, 2W | 3M, 2W | 5M, 1L | 9M, 6W | 8.11 | 0.23 |
| Handedness | 5R | 3R, 1M, 1L | 5R, 1L | 14R, 1L | 6.03 | 0.42 |
| Chronicity | 21.6 (10.2) | 14 (4.7) | 9.8 (1.9) | – | 4.73 | 0.03 |
| WAIS-III FSIQ | 110 (21.1) | 102 (8.9) | 108 (20.6) | – | 0.50 | 0.62 |
| BDI | 4 (2.7) | 5 (2.2) | 6 (6.1) | – | 0.04 | 0.96 |
| PANAS positive | 27.2 (7.1) | 38.8 (6.6) | 33.2 (5.4) | 33.3 (5.4) | 3.26 | 0.04 |
| PANAS negative | 11.6 (3.05) | 12.2 (2.8) | 10.5 (0.8) | 11.3 (1.8) | 0.65 | 0.59 |
| Racial contact | 13.8 (17.6) | 19 (16.9) | 18.7 (17.1) | 15.7 (15.1) | 0.14 | 0.94 |
| Stereotypes | 11.6 (4.3) | 11.2 (3.4) | 12.1 (2.7) | 11.7 (4.3) | 0.06 | 0.98 |
| Symbolic racism | 0.5 (0.2) | 0.4 (0.1) | 0.3 (0.2) | 0.4 (0.2) | 1.30 | 0.30 |
Note: Age is in years at time of testing. Education is education in years of formal schooling. Class is self-reported social class (W = working class, M = middle class). Handedness reports dominant hand (R = right, M = Mixed, L = left). Chronicity is the time between lesion onset and completion of the present experiment, in years. WAIS-III, Wechsler Adult Intelligence Scale-III scores (FSIQ = full-scale IQ), 80–89 is low average, 90–109 is average, 110–119 is high average, 120+ is superior). BDI, Beck Depression Inventory, a measure of baseline mood (raw scores reported. According to the BDI-II manual, ‘Nondepressed’ individuals had mean BDI-II scores of 7.7 (s.d. 5.9), whereas ‘mildly depressed’, ‘moderately depressed’, and ‘severely depressed’ individuals had mean BDI-II scores of 19.1 (s.d. 5.7), 27.4 (s.d. 10.0), and 33.0 (s.d. 12.0), respectively (Beck, Steer, and Brown 1996).
Mixed-effects regression
Results from the full models are reported in Table A4 of the online Supplementary, and the proportions reporting desirability-related and distressful emotions collapsed across conditions are reported in Figure 2. Looking at the desirability-related emotions, we see that race has no significant main effects, while pictures of middle and upper-class compared to lower-class increased ratings of desirability-related emotions significantly by 0.50 (P < 0.01) and 0.35 (P < 0.01) units. Lesion types did not have any significant main effects. None of the race X class, race X lesion and class X lesion interaction effects were statistically significant but the three-way Black X middle-class X vmPFC interaction was significant and negative. In order to clarify what is driving this effect, we have conducted three posthoc contrasts. The first two contrast looked at the differences between the vmPFC and the normal/BDC group on their ratings of middle-class racial group. These tests revealed no significant results. A third test looked at whether or not the vmPFC group’s relative ratings of racial groups within the middle-class condition differed from that of the normal/BDC group. This contrast revealed that the vmPFC group’s ratings of White vs Black middle-class was more positive and significantly greater than that of the normal/BDC (uncorrected P = 0.0006, Bonferroni corrected P for three contrasts < 0.01).
Fig. 2.
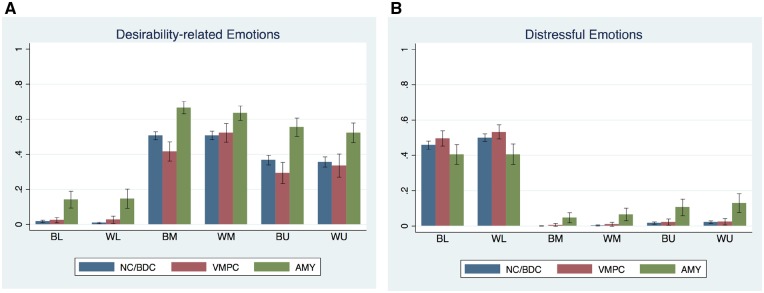
Proportions of reporting desirability-related and distressful emotions collapsed across conditions for each group. Note: Error bars indicate 95% confidence intervals. BL = Black lower class, WL = White lower class, BM = Black middle class, WM = White middle class, BU = Black upper-class, WU = White upper-class.
When we turn to distressful emotions, we see that race had a significant main effect; pictures of Black people were rated less negatively than Whites (P < 0.001). Pictures of middle and upper-class compared to lower-class were rated more negatively (P < 0.001). Lesion types had no significant effects. Black by middle-class and Black by upper-class interaction effects were significant and in positive direction (P < 0.01). Race by lesion interactions were not significant. Amygdala by middle-class and Amygdala by upper-class interactions were significant and positive (P < 0.001). This suggests that the Amygdala group’s ratings of middle and upper-class groups were more positive than the other lesion groups. When we look at the three-way race by class by lesion interaction, on the other hand, we see that the interaction effects are in the negative direction and significant for the Black by upper-class X Amygdala interaction. When we unpack this interaction effect with two posthoc contrasts, we find that the Amygdala group reported greater distressful emotions for the White upper-class than the normal/BDC group (uncorrected P = 0.0291, Bonferroni corrected P for two contrasts < 0.1).
Combining all desirability-related and distressful emotions together potentially obscures nuance, such as the distinction between moral and basic emotions as they relate to contemporary racial evaluations (Lamont, 2000; Sears and Henry, 2003; Neville et al., 2013). Thus, we conducted post-investigations with two moral emotions (one desirability-related and one distressful)—pride and disgust—and two basic emotions (similarly one desirability-related and one distressful)—happiness and anger—known to be important for racial evaluations and out-group stigma (Rogers and Prentice-Dunn, 1981; Harris and Fiske, 2007; Markus 2008). We fitted multilevel mixed-effects logistic regression models on the single emotions (a total of four models) to estimate the effects of race, class and lesion types and then followed-up with contrasts to examine potential racial differences on the ratings of these emotions within each class condition. The amygdala patients showed some significant differences for the lower-class and the vmPFC patients for the middle-class in both basic and moral emotions. The amygdala patients reported higher happiness (uncorrected P = 0.021, Bonferroni corrected P for six contrasts < 0.151) and lower disgust (uncorrected P = 0.0364, Bonferroni corrected P for six contrasts < 0.15) when viewing Black vs White lower-class compared to the normal/BDC group. The vmPFC patients reported less happiness (uncorrected P = 0.0181, Bonferroni corrected P for six contrasts < 0.12) and less pride (uncorrected P = 0.0025, Bonferroni corrected P for six contrasts < 0.05) in response to the pictures of Black vs White middle-class compared to the normal/BDC group.
To summarize, while these effects are small, they suggest an interesting pattern. When focused on racial members in different socio-economic positions, the vmPFC differences were more likely to pertain to desirability-related emotions and the middle-class group, while the amygdala effects were largely confined to distressful emotions and the upper-class racial out-groups. Our findings extend previous research on the vmPFC’s role in empathy and personal moral judgments (Shamay-Tsoory et al., 2005; Ciaramelli et al., 2007; Leopold et al., 2012) by showing that the vmPFC lesions can also bias subtle social emotional differences within socially valued categories (i.e. the middle-class). Additionally, results pertaining to the amygdala lesions are concordant with research showing that the amygdala is related to emotional vigilance, especially to ambiguous external stimuli (Davis and Whalen, 2001) and thus to racial evaluations in socially ambivalent out-group categories (i.e. lower-class and upper-class).
Experiment 2: fMRI
Participants
Fifteen right-handed adults with no history of neurological or psychiatric disorders were recruited using the same age, education and income screening criteria as the previous sample. Participants were additionally pre-screened for metal on or in their body and for anxiety attacks, panic disorder, claustrophobia, pregnant (or trying), or breast feeding. Data from two subjects were discarded because of excessive head movement during the MRI session (if the Euclidian norm of motion derivatives were >4 mm in >10% of the data). The final sample size is 13 subjects (7 male, 6 female, mean age = 47.2 s.d. = 7.6, mean education = 14.5 s.d. = 2.2).
Stimuli and procedure
A subset of the pictures from the neuropsychological experiment (excluding eight pictures in order to keep the blocks balanced), using a pseudorandomized block design of eight runs and eight blocks were shown inside the scanner. Each block consisted of three pictures, 6 s per picture. Eight blocks (a total of 24 pictures) were used for each condition. All pictures were presented again on a computer immediately after the imaging session. Subjects evaluated the pictures on eight emotions, as well as the scales used in the lesion experiment.
Behavioral analysis
Similar to the first experiment, we first conducted a principal components factor analysis, which suggested a two-factor solution (see online Supplementary Table A5). Thus, we averaged happiness, pride and envy into one desirability-related emotion variable (Cronbach’s Alpha = 0.84) and anger, sadness, fear, disgust and pity into a distressful emotion variable (Cronbach’s Alpha = 0.76). We then analyzed the desirability-related and distressful emotion scores separately by fitting multilevel mixed-effects regression models.
fMRI image acquisition and analysis
Imaging was conducted using a Siemens TIM Trio 3T scanner and a 12-channel head coil. Anatomic data consisted of volumetric T1-weighted MP-RAGE images (repetition time (TR)/echo time (TE)/inversion time (TI): 2530/3.09/900 ms, FOV: 256 mm×256 mm×240 mm, matrix: 256×256×240, slice thickness: 1 mm, flip angle: 10°). Functional data were acquired with blood oxygen level dependent contrast echo-planar imaging (TR/TE of 2000/30 ms, FOV: 220 mm, matrix: 64×64, slice thickness/gap: 3.5/0.525 mm, 31 transversal slices, flip angle: 90°). Data were processed with AFNI (Cox, 1996). Each functional run (echo-planar image) was composed of 155 temporal volumes (number of repetitions). The two first volumes corresponding to the stabilization period of the magnetic signal were not considered for further analysis.
Preprocessing of echo-planar imaging data included the following: (i) removal of large signal deviations of 2.5 s.d. or greater from the mean, (ii) slice-time correction, (iii) co-registration with the anatomical images and transformation to the TT-N27 atlas—aka Colin brain—within AFNI (3-mm isotropic voxels), (iv) 3-dimensional volume registration (all EPI runs were coregistered with the 5th volume from the first imaging run using a heptic polynomial interpolation method), (v) smoothing with a Gaussian spatial filter of 4 mm, and (vi) scaling of blood oxygen level–dependent signal intensity to percentage of signal change using each subject's voxelwise time series mean as a baseline. A deconvolution analysis extracted a hemodynamic response function for each subject. The effects of the conditions were modeled by box-car regressors convolved with the hemodynamic response function for 18 s for blocks of each trial type. Motion correction parameters were included as nuisance covariates and TRs with motion derivatives exceeding the Euclidian norm of 0.4 mm were censored in deconvolution analysis.
Group-level analyses on the hemodynamic response estimates were conducted in two ways: (i) Group-level ANOVA and (ii) Region of Interest (ROI) analysis. First, a two-factor mixed-effects ANOVA in which conditions were the fixed (within subjects) factors and percentage BOLD signal changes from each participant was the random (between-subjects) factor applied. A group-level intersection mask with 70% overlap, obtained from the normalized and co-registered subject-level union masks, was applied to whole-brain group-level analyses. Second, we conducted separate ANOVAs applying ROI masks for the MPFC and the amygdala. The ROI mask for the amygdala was generated from the Eickhoff–Zilles cytoarchitectonic probabilistic atlas (AFNI’s CA_N27_ML Atlas). The ROI in the MPFC was generated using a 10-mm sphere centered at coordinates (2 −48 −7 in RAI coordinates) based on previous research (Harris and Fiske, 2006; Schreiber and Iacoboni, 2012). All reported statistics were family-wise corrected for multiple comparisons with Monte Carlo simulations for an alpha-level of 0.05 (Forman et al. 1995; Ward 2002). Using the parameters of our data set (FWHM = 5.8), running 10 000 iterations over the brain-only mask used in whole-brain group-level analyses indicated that for a corrected cluster-wise activation threshold of P < 0.05, the minimum cluster sizes of 37 and 24 should be considered for voxel-wise thresholds of P < 0.01, P < 0.005, respectively. For a corrected alpha-level of 0.05 using a voxel-wise threshold of 0.05, running Monte Carlo simulation on ROI masks also indicated the minimum cluster sizes of 20 and 9 for the MPFC, and the left and right amygdala, respectively.
Results
Demographic characteristics
All respondents were adults (average age = 47, s.d. = 7.58, 6 women, 7 men) averaged some college education (14.5 years, s.d. = 2.18). Five subjects reported to be middle-class, seven reported to be working-class and one participant reported lower-class status. Subjects had an average positive affect score of 29.5 (s.d. = 6.52) and negative affect score of 11.1 (s.d. = 0.86), which are well within the normal thresholds of affect (Watson and Clark 1999). The average racial contact score is 14.6 (s.d. = 13.71), stereotype assessment score is 10 (out of 24, s.d. = 6.06) and symbolic racism score is 0.40 (out of 1, s.d. = 0.20).
Behavioral results
Results from the multilevel mixed-effects logistic regression models are reported in Table A6 of the online Supplementary. Results showed that race had no significant main effects on desirability-related emotions, while pictures of middle and upper-class people elicited greater desirability-related emotional reports. The only significant interaction term indicated that pictures of Black middle-class people were received with less desirability-related emotions than White middle-class (P < 0.01). Race also had no significant main effects on distressful emotions and respondents reported reduces distressful emotions in response to pictures of middle and upper-class people. Race X social class interactions had no significant effects on distressful emotions. It should be noted that the picture-rating task took place after the functional imaging, thus subjects were likely aware of the purpose of the experiment by this time, potentially explaining lack of significant effects.
Whole-brain fMRI results
The areas of significant activation for each condition from the whole-brain ANOVA are reported in Table A7 of the online Supplementary. In general, brain regions associated with visuospatial processing and memory like lingual gyri, cuneus, precuneus, supplementary motor area, temporal gyri and superior parietal lobules were activated across all or most conditions. Brain regions related to emotional processing such as the anterior and mid cingulate cortices and the inferior parietal lobule (Bush, Luu and Posner, 2000; Canli et al., 2004) were activated mostly in response to human conditions (vs non-humans). Furthermore, areas related to distressful emotions processing and regulation like the inferior frontal gyri and the insular lobes (Ochsner et al., 2004; Goldin et al., 2008) showed activation in relation to out-group class conditions like pictures of White and Black upper and lower-class people but not the middle class.
To further identify differences in the activation of brain regions across race conditions, we included three specific contrasts in the ANOVA (White lower vs Black lower, White middle vs Black middle, White upper vs Black upper). The contrast of middle-class Whites vs Blacks was the only activation cluster that survived the significance thresholds. Significant activation in the left superior orbital gyrus was revealed for White middle-class vs Black middle-class (x, y, z =11, −26, −16, t = 4.603, P corrected < 0.05). As the orbital gyri are contained in the ventromedial prefrontal cortex (MacPherson et al. 2002), this suggests that the vmPFC is involved in racial evaluations favoring White middle-class vs Black. None of the other race contrasts (e.g. Black low vs White low) produced a statistically significant difference.
ROI results
Results of the MPFC ROI analyses converge with the whole brain analysis and previous literature (see Table A8 of the online Supplementary for the main condition effects). The only significant activation clusters were for White and Black upper social classes, and the White middle and lower-class (but not Black) conditions (corrected P < 0.05). For the race contrasts, there was greater MPFC activation while viewing pictures of White middle-class vs Black middle-class people (t = 3.247, P corrected < 0.05, see Table 3 and Figure 3). The only other significant contrast was of viewing pictures of humans (collapsed across all race and class groups) vs non-humans (pleasant and unpleasant collapsed) (x = −2, y = −53, z = 16, t = 7.267, P corrected < 0.05).
Table 3.
Contrast results from the ROI analysis
| Volume mm3 | x | y | z | Maximal t-score | |
|---|---|---|---|---|---|
| MPFC | |||||
| White mid.>Black mid. | 31 | −2 | −50 | −16 | 3.247 |
| Human>Non-human | 85 | −2 | −53 | −16 | 7.267 |
| RAMY | |||||
| White lower-class>Black lower class | 13 | −23 | 2 | −10 | −5.547 |
| LAMY | |||||
| Human>Non-human | 9 | 26 | 5 | −13 | −3.782 |
Note: Peak activation. Talairach coordinates x = Right-to-Left, y = Anterior-to-Posterior, and z = Inferior-to-Superior (RAI). Contrasts tested: White lower vs Black lower, White middle vs Black middle, White upper vs Black upper, Human vs Non-human. All reported statistics survived family-wise corrections for multiple comparisons with Monte Carlo simulations for an alpha-level of 0.05. Uncorrected alpha level for the White middle vs Black middle contrast is 0.06, the rest of the contrasts are at the level of 0.05.
Fig. 3.
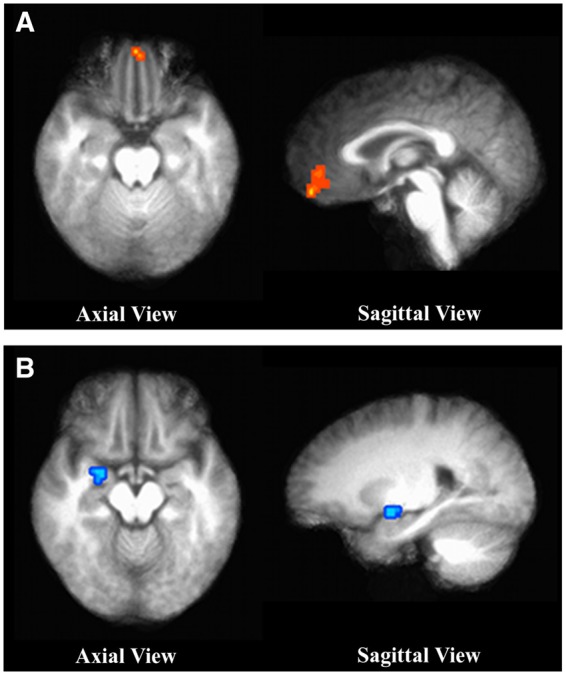
(A) Region of the MPFC that was more active during the presentation of White vs Black middle-class pictures. X, Y, Z Talairach coordinates (RAI): −2, −50, −16. Corrected P< 0.05. t value: 3.247. (B) Region of the right amygdala that was less active during the presentation of White vs Black lower-class pictures. X, Y, Z Talairach coordinates (RAI): −23, 2, −10.
These findings align with previous research indicating the involvement of the vmPFC in personal and emotional moral evaluations, perspective-taking or ability to empathize with others, reward and pleasure mechanisms (Bechara et al., 2000; Kringelbach and Berridge, 2009) as well as activation of the subgenual cingulate areas with kin-related cohesion and a lack of activation in dehumanization of moral out-groups (Harris and Fiske, 2006). However, rather than being differentially involved in evaluations of racial groups across all class conditions, our results show that the significant differences in the vmPFC activation were specific to the middle-class group.
Results from the amygdala ROI revealed that while the right amygdala was activated in all conditions except the non-human pleasant pictures, the left amygdala was activated in all conditions (corrected P < 0.05). These findings are consistent with the hypothesis that the amygdala has a general role related to emotional arousal (see Phan et al., 2002). Looking at the contrasts between these conditions, right amygdala activation was significantly different only between White and Black lower-class conditions. There was lower right amygdala activation for the White vs Black lower-class (t = −5.547, corrected P < 0.05) (see Figure 3). On the other hand, the only significant left amygdala activation difference was the contrast between humans and non-humans. There was decreased activity for the conditions with pictures of humans compared to those with non-humans (t = −3.782, P corrected < 0.05). These findings are in line with previous research showing that increased right amygdala activation during unconscious evaluations of masked or hidden stimuli (Morris et al., 1998, 1999) as well as when viewing faces of Blacks vs Whites (Lieberman et al., 2005; Ronquillo et al., 2007). However, our results show the right amygdala is differentially involved in racial evaluations, but only observing stimuli from the lower-class conditions.
Discussion
Our study investigated the intersection of social class and race. Complementary neuropsychological and functional imaging experiments demonstrated the involvement of the vmPFC in perceiving racial differences within the middle-class, and the amygdala in emotional responses to the upper and lower class. Specifically, results from the neuropsychological experiment revealed the following: (i) the importance of moral emotional processing in racial evaluations, and (ii) the potential recruitment of distinct brain regions in class-based racial evaluations: the vmPFC for the middle-class and the amygdala for the lower-class and upper-class. Findings from the fMRI experiment also supported these results, with significant vmPFC activation in response to White vs Black middle-class and significant amygdala deactivation in response to White vs Black lower class.
Previous research indicates the vmPFC is crucial for social emotions and moral empathy as well as reward processing and pleasure (Damasio, 1994; Greene and Haidt, 2002; Berridge and Kringelbach, 2008). However, the role of mPFC and more particularly vmPFC in race evaluations is poorly understood. While several studies have linked this region to mentalizing about others and empathy (Frith and Frith, 2001; Gallagher and Frith, 2003), few studies focused on its involvement in race-evaluations. Our study offers novel evidence in favor of the thesis that the vmPFC’s role for racial evaluations is largely confined to the more positively valued and prized groups, potentially through regulating desirability-related emotions or representing values assigned to these social groups (Kringelbach and Rolls, 2004; Mitchell et al., 2006; Volz et al., 2009; Krienen et al., 2010). Interacting with both Black and White middle-class people likely elicits desirability-related and moral emotions (e.g. happiness, pride). However, as implicated in our findings, social appraisals within the middle-class group might still be more favorable towards Whites. While this view fits with sociological research reporting middle-class Blacks are still discriminated against despite their desirable class status (Feagin, 1991; Feagin and Sikes, 1994), it also extends the field by suggesting racial evaluation processes might be operating differently for middle-class members.
Results from both experiments also show that the amygdala plays a role in racial evaluations, but particularly for out-group class conditions. These results suggest that rather than automatically categorizing humans into different racial groups, the amygdala might be encoding other socially valued properties from the facial region, complementing previous research indicating that individuals with amygdala lesions have impaired startle reflex, impaired recognition of emotional face expressions (especially fear) and social judgments like detecting trustworthiness of others (Adolphs et al., 1994, 1998; Angrilli et al., 1996; Boucsein et al., 2001).
Moreover, these findings help shed some light on contradictory results from previous literature. While several studies found increased amygdala activation for racial out-groups (Hart et al., 2000; Phelps et al., 2000; Lieberman et al., 2005), a lesion study revealed no explicit or implicit measures of racial attitudes for patients with amygdala damage (Phelps et al., 2003). Furthermore, an experimental task in which participants were shown norm-violating (e.g. gang members, prison inmates) and norm-consistent (families, teachers) pictures of Blacks and Whites, Schreiber and Iacoboni (2012) found that amygdala activation is related to norm-violation rather than race evaluations. These discrepancies suggest that facial pictures of Black or White individuals isolated from distinguishing social context cues are not good indicators of emotional differences. Relying solely on these measures might miss important, emotional components of racial attitudes. By taking into account a more nuanced system of racial evaluations that includes positive appraisals and emotions triggered by class markers, future research can move beyond viewing social bias as a unidirectional animosity towards all members of a group.
Our study has several limitations. First, our stimuli consisted of a constrained set of social class and racial groups in order to minimize potential confounds. Future research could consider a more nuanced approach that extends the inquiry to evaluations of other class groups (working class, upper-middle class) as well as other racial and ethnic groups. Second, we cannot draw conclusions about the differential effects of the emotions under investigation because we employed an analytical strategy that combined these emotions into two latent clusters. Third, the brain regions of interest in this study are susceptible to signal loss and image distortion due to their location near air or bone/tissue interfaces (Merboldt et al., 2001; Deichmann et al., 2003). We attempted to minimize such artifacts while acquiring whole brain coverage by using a 3.5-mm slice thickness. However, caution in interpreting our results is urged. Newer higher-channel count head coils coupled with multi-band imaging would allow further minimization of slice thickness and resulting susceptibility artifacts. Fourth, these studies employed relatively small sample sizes posing a challenge to the generalizability of the findings. However, converging findings from neuropsychological and functional imaging approaches help strengthen our conclusions. Furthermore, we recruited adults older than age 30 years from the local population, avoiding possible drawbacks of sampling techniques used frequently in psychological and functional imaging experiments with undergraduate student populations (see Henrich, Heine, and Norenzayan, 2010 for a critique).
Finally, in order to keep the samples comparable across neuropsychological and fMRI studies, our samples included mainly middle- and working-class White individuals. Sample homogeneity limits the interpretation of our results such that we cannot conclude whether differences in brain response are due to group identification or stereotypes. For example, greater vmPFC activation in response to pictures of White vs Black middle-class people might be due to in-group favoritism or knowledge about positive group stereotypes. However, based on previous literature, we suggest that while both mechanisms are likely operating simultaneously, group identification processes might recruit the vmPFC and the stereotype-related activation might be associated with the amygdala. Previous studies show that the vmPFC is related to personal (vs impersonal) moral judgments (Greene et al., 2001; Ciaramelli et al., 2007), self and in-group related information (Kim and Johnson, 2015), and sensitivity to in-group harm (Cikara et al., 2010; Molenberghs et al., 2014). Amygdala activity, conversely, is correlated with norm violation rather than race, and is observed not only in Whites but also Blacks (Lieberman et al., 2005) and is absent in childhood and does not emerge until adolescence (Telzer et al., 2013). Therefore, we conclude that two distinct mechanisms, one related to group identification engaging the vmPFC for the middle-class and another more pertinent to stereotypes recruiting the amygdala for the upper and lower-class positions, potentially underlie class-based racial evaluations.
Funding
This work was supported in part by a McDonnell Foundation Collaborative Action Award [#220020387 to D.T.], the Social Science Research Council, University of Iowa MR Research Facility, Executive Council for the Graduate and Professional Students and Department of Sociology at the University of Iowa.
Supplementary data
Supplementary data are available at SCAN online.
Supplementary Material
Acknowledgements
We would like to thank Matthew Andersson, Mary Campbell, Jeremy Freese, Jennifer Glanville, Gunes Sevinc and Jessica Wellburn for helpful feedback on earlier drafts of this paper. We would also like to acknowledge Bradley Taber-Thomas for valuable advice on research strategy, Joel Bruss for assisting the analyses of neuroanatomical data, Ruth Henson and Keary Saul for assisting subject recruitment, Ben Earnhart and Nick Jones for technical support, and Beyon Miloyan, Thelma Moss, Sarah Nutter and Sarah Purcell for their research assistance.
Conflict of interest. None declared.
Footnotes
Bonferroni corrections tend to be very conservative especially as the number of tests increase. In the case of our comparisons, an alpha level of 0.017 and 0.025 are required for even to reach a more liberal corrected alpha level of 0.1 for six and four contrasts, respectively. This is why we are reporting uncorrected significant results with the corresponding corrected alpha values in order to reduce false negatives.
References
- Adolphs R. (2009). The social brain: neural basis of social knowledge. Annual Review of Psychology, 60, 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R., Tranel D., Damasio H., Damasio A.R. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372, 669–72. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Tranel D., Damasio A.R. (1998). The human amygdala in social judgment. Nature 393, 470–4. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Russell J.A., Tranel D. (1999). A role for the human amygdala in recognizing emotional arousal from unpleasant stimuli. Psychological Science, 10(2),167–71. [Google Scholar]
- Angrilli A., Mauri A., Palomba D., et al. (1996). Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain, 119(6),1991–2004. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R. (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex, 10 (3),295–307. [DOI] [PubMed] [Google Scholar]
- Beck, A.T., Steer, R.A., Brown, G.K. (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation.
- Berridge K.C., Kringelbach M.L. (2008). Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology, 199, 457–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Silva E. (2010). Racism without Racists: Color-Blind Racism and the Persistence of Racial Inequality in the United States. Lanham, MD: Rowman and Littlefield. [Google Scholar]
- Boucsein K., Weniger G., Mursch K., Steinhoff B.J., Irle E. (2001). Amygdala lesion in temporal lobe epilepsy subjects impairs associative learning of emotional facial expressions. Neuropsychologia, 39(3),231–6. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6),215–22. [DOI] [PubMed] [Google Scholar]
- Canli T., Sivers H., Thomason M.E., Whitfield-Gabrieli S., Gabrieli J.D., Gotlib I.H. (2004). Brain activation to emotional words in depressed vs healthy subjects. Neuroreport, 15(17),2585–8. [DOI] [PubMed] [Google Scholar]
- Chiao J.Y., Iidaka T., Gordon H.L., et al. (2008). Cultural specificity in amygdala response to fear faces. Journal of Cognitive Neuroscience, 20(12),2167–74. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E., Muccioli M., Ladavas E., Pellegrino G. (2007). Selective deficit in personal moral judgment following damage to ventromedial prefrontal cortex. Social Cognitive and Affective Neuroscience, 2(2),84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikara, M., Farnsworth, R. A., Harris, L. T., Fiske, S. T. (2010). On the wrong side of the trolley track: Neural correlates of relative social valuation. Social cognitive and affective neuroscience, 5(4), 404–13. [DOI] [PMC free article] [PubMed]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical research, 29(3), 162–73. [DOI] [PubMed]
- Cunningham W.A., Johnson M.K., Raye C.L., Gatenby J.C., Gore J.C., Banaji M.R. (2004). Separable neural components in the processing of Black and White faces. Psychological Science, 15(12),806–13. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. (2004). The emotional brain. Nature Reviews Neuroscience, 5, 582–9. [DOI] [PubMed] [Google Scholar]
- Damasio A.R. (1994). Descartes' Error: Emotion, Reason, and the Human Brain. New York: Avon Books. [Google Scholar]
- Damasio H., Grabowski T.J., Tranel D., Hichwa R.D., Damasio A.R. (1996). A neural basis for lexical retrieval. Nature, 380, 499–505. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6, 13–34. [DOI] [PubMed] [Google Scholar]
- Deichmann R., Gottfried J.A., Hutton C., Turner R. (2003). Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage, 19(2),430–41. [DOI] [PubMed] [Google Scholar]
- Feagin J. (1991). The continuing significance of race: antiblack discrimination in public places. American Sociological Review, 56, 101–16. [Google Scholar]
- Feagin J.R., Sikes M.P. (1994). Living with Racism: The Black Middle-Class Experience. Boston, MA: Beacon Press. [Google Scholar]
- FeldmanHall O., Dalgleish T., Thompson R., Evans D., Schweizer S., Mobbs D. (2012). Differential neural circuitry and self-interest in real vs hypothetical moral decisions. Social Cognitive and Affective Neuroscience, 7(7),743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske S.T. (2009). Social cognition In: Sander D., Scherer K.R., editors. Oxford Companion to Emotion and the Affective Sciences (pp. 371–3). Oxford: Oxford University Press. [Google Scholar]
- Forman, S. D., Cohen, J. D., Fitzgerald, M., Eddy, W. F., Mintun, M. A., Noll, D. C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster?size threshold. Magnetic Resonance in medicine, 33(5), 636–47. [DOI] [PubMed]
- Frith U., Frith C. (2001). The biological basis of social interaction. Current Directions in Psychological Science, 10(5),151–5. [Google Scholar]
- Gallagher H.L., Frith C.D. (2003). Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences, 7(2),77–83. [DOI] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6),577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J., Haidt J. (2002). How (and where) does moral judgment work? Trends in Cognitive Sciences, 6(12),517–23. [DOI] [PubMed] [Google Scholar]
- Greene J.D., Sommerville R.B., Nystrom L.E., Darley J.M., Cohen J.D. (2001). An fMRI investigation of emotional engagement in moral judgment. Science, 293, 2105–8. [DOI] [PubMed] [Google Scholar]
- Greenwald A.G., Banaji M.R. (1995). Implicit social cognition: attitudes, self-esteem, and stereotypes. Psychological Review, 102(1),4–27. [DOI] [PubMed] [Google Scholar]
- Gutsell J.N., Inzlicht M. (2012). Intergroup differences in the sharing of emotive states: neural evidence of an empathy gap. Social Cognitive and Affective Neuroscience, 7(5),596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidt J. (2007). The new synthesis in moral psychology. Science, 316, 998–1002. [DOI] [PubMed] [Google Scholar]
- Haidt J. (2008). Morality. Perspectives on Psychological Science, 3(1),65–72. [DOI] [PubMed] [Google Scholar]
- Hamann S.B., Ely T.D., Hoffman J.M., Kilts C.D. (2002). Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychological Science, 13(2),135–41. [DOI] [PubMed] [Google Scholar]
- Harris L.T., Fiske S.T. (2006). Dehumanizing the lowest of the low: neuroimaging responses extreme out-groups. Psychological Science, 17, 847–53. [DOI] [PubMed] [Google Scholar]
- Harris L.T., Fiske S.T. (2007). Social groups that elicit disgust are differentially processed in MPFC. Social Cognitive and Affective Neuroscience, 2, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A.J., Whalen P.J., Shin L.M., McInerney S.C., Fischer H., Rauch S.L. (2000). Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport, 1, 2351–2355. [DOI] [PubMed] [Google Scholar]
- Haslam N. (2006). Dehumanization: an integrative review. Personality and Social Psychology Review, 10, 252–64. [DOI] [PubMed] [Google Scholar]
- Henrich, J., Heine, S. J., Norenzayan, A. (2010). Most people are not WEIRD. Nature, 466(7302), 29. [DOI] [PubMed]
- Hochschild J.L., Scovronick N. (2004). The American Dream and the Public Schools. Oxford:Oxford University Press. [Google Scholar]
- Horvat E.M., Weininger E.B., Lareau A. (2003). From social ties to social capital: class differences in the relations between schools and parent networks. American Educational Research Journal, 40(2),319–51. [Google Scholar]
- Hout M. (2008). How class works: objective and subjective aspects of class since the 1970s In: Lareau A., Conley D., editors. Social Class: How Does It Work? (pp. 25–64). New York: Russell Sage Foundation. [Google Scholar]
- Hunt M.O. (2007). African American, Hispanic, and white beliefs about black/white inequality, 1977-2004. American Sociological Review, 72(3),390–415. [Google Scholar]
- Kim K., Johnson M.K. (2015). Activity in ventromedial prefrontal cortex during self-related processing: positive subjective value or personal significance? Social Cognitive and Affective Neuroscience, 10(4),494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Young L., Adolphs R., et al. (2007). Damage to the prefrontal cortex increases utilitarian moral judgements. Nature, 446, 908–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen F.M., Tu P., Buckner R.L. (2010). Clan mentality: evidence that the medial prefrontal cortex responds to close others. The Journal of Neuroscience, 30(41),13906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L., Berridge K.C. (2009). Towards a functional neuroanatomy of pleasure and happiness. Trends in Cognitive Sciences, 13, 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L., Rolls E.T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72(5),341–72. [DOI] [PubMed] [Google Scholar]
- Lamont M. (1992). Money, Morals, and Manners: The Culture of the French and the American Upper-Middle Class. Chicago: University of Chicago Press. [Google Scholar]
- Lamont M. (2000). The Dignity of Working Men: Morality and the Boundaries of Race. Class and Immigration. Cambridge, MA: Harvard University Press. [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (1995). International Affective Picture System (IAPS). Bethesda, MD: National institute of Mental Health Center for the Study of Emotion and Attention. [Google Scholar]
- LeDoux J.E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–84. [DOI] [PubMed] [Google Scholar]
- Leopold A., Krueger F., dal Monte O., et al. (2012). Damage to the left ventromedial prefrontal cortex impacts affective theory of mind. Social Cognitive and Affective Neuroscience, 7(8),871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyens J. Ph., Cortes B., Demoulin S., et al. (2003). Emotional prejudice, essentialism, and nationalism: the 2002 Tajfel lecture. European Journal of Social Psychology, 33, 703–17. [Google Scholar]
- Lieberman M.D., Hariri A., Jarcho J.M., Eisenberger N.I., Bookheimer S.Y. (2005). An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nature Neuroscience, 8(6),720–3. [DOI] [PubMed] [Google Scholar]
- MacPherson S.E., Phillips L.H., Sergio D.S. (2002). Age, executive function and social decision making: a dorsolateral prefrontal theory of cognitive aging. Psychology and Aging, 17(4),598–609. [PubMed] [Google Scholar]
- Madon S., Guyll M., Aboufadel K., et al. (2001). Ethnic and national stereotypes: the Princeton trilogy revisited and revised. Personality and Social Psychology Bulletin, 27(8),996–1010. [Google Scholar]
- Markus H.R. (2008). Pride, prejudice, and ambivalence: toward a unified theory of race and ethnicity. American Psychologist, 63(8),651.. [DOI] [PubMed] [Google Scholar]
- Merboldt K.D., Fransson P., Bruhn H., Frahm J. (2001). Functional MRI of the human amygdala? Neuroimage, 14(2),253–7. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Macrae C.N., Banaji M.R. (2006). Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron, 50(4),655–63. [DOI] [PubMed] [Google Scholar]
- Molenberghs P., Gapp J., Wang B., Louis W.R., Decety J. (2014). Increased moral sensitivity for outgroup perpetrators harming ingroup members. Cerebral Cortex, 26(1),225–33. [DOI] [PubMed] [Google Scholar]
- Moll J., de Oliveira-Souza R., Eslinger P.J., et al. (2002). The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. The Journal of Neuroscience, 22(7),2730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., de Oliveira-Souza R., Moll F.T., et al. (2005). The moral affiliations of disgust: a functional MRI study. Cognitive and Behavioral Neurology, 18(1),68–78. [DOI] [PubMed] [Google Scholar]
- Morris J.S., Öhman A., Dolan R.J. (1998). Conscious and unconscious emotional learning in the human amygdala. Nature, 393, 467–70. [DOI] [PubMed] [Google Scholar]
- Morris J.S., Öhman A., Dolan R.J. (1999). A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences, 96, 1680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville H.A., Awad G.H., Brooks J.E., Flores M.P., Bluemel J. (2013). Color-blind racial ideology: theory, training, and measurement implications in psychology. American Psychologist, 68(6),455.. [DOI] [PubMed] [Google Scholar]
- Nosek B.A., Smyth F.L., Hansen J.J., et al. (2007). Pervasiveness and correlates of implicit attitudes and stereotypes. European Review of Social Psychology, 18(1),36–88. [Google Scholar]
- Nucci L., Turiel E. (2000). The moral and the personal: sources of social conflicts In: Nucci L.P., Saxe G.B., Turiel E., editors. Culture, Thought, and Development (pp. 115–37). Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage, 23(2),483–99. [DOI] [PubMed] [Google Scholar]
- Pager D., Shepherd H. (2008). The sociology of discrimination: racial discrimination in employment, housing, credit, and consumer markets. Annual Review of Sociology, 34, 181–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in Pet and fMRI. Neuroimage, 16, 331–48. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., O'Connor K.J., Cunningham W.A., et al. (2000). Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience, 12(5),729–38. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Cannistraci C.J., Cunningham W.A. (2003). Intact performance on an indirect measure of race bias following amygdala damage. Neuropsychologia, 41, 203–8. [DOI] [PubMed] [Google Scholar]
- Prehn K., Wartenburger I., Mériau K., et al. (2008). Individual differences in moral judgment competence influence neural correlates of socio-normative judgments. Social Cognitive and Affective Neuroscience, 3(1),33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. (1999). Prefrontal cortical networks related to visceral function and mood. Annals of the New York Academy of Sciences, 877, 383–96. [DOI] [PubMed] [Google Scholar]
- Richeson J.A., Baird A.A., Gordon H.L., et al. (2003). An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience, 6(12),1323–8. [DOI] [PubMed] [Google Scholar]
- Richeson J.A., Todd A.R., Trawalter S., Baird A.A. (2008). Eye-gaze direction modulates race-related amygdala activity. Group Processes & Intergroup Relations, 11(2),233–46. [Google Scholar]
- Rogers R.W., Prentice-Dunn S. (1981). Deindividuation and anger-mediated interracial aggression: unmasking regressive racism. Journal of Personality and Social Psychology, 41(1),63. [Google Scholar]
- Ronquillo J., Denson T.F., Lickel B., Lu Z., Nandy A., Maddox K.B. (2007). The effects of skin tone on race-related amygdala activity: an fMRI investigation. Social Cognitive and Affective Neuroscience, 2, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer A. (2005). The Moral Significance of Class. Cambridge: Cambridge University Press. [Google Scholar]
- Schneider M., Buckley J. (2002). What do parents want from schools? Evidence from the Internet. Educational Evaluation and Policy Analysis, 24(2),133–44. [Google Scholar]
- Schreiber D., Iacoboni M. (2012). Huxtables on the brain: an fMRI study of race and norm violation. Political Psychology, 33(3),313–30. [Google Scholar]
- Schuman H., Steeh C., Bobo L., Krysan M. (1997). Racial Attitudes in America: Trends and Interpretations. Cambridge, MA: Harvard University Press. [Google Scholar]
- Schwartz C.R., Mare R.D. (2005). Trends in educational assortative marriage from 1940 to 2003. Demography, 42(4),621–46. [DOI] [PubMed] [Google Scholar]
- Sears D.O., Henry P.J. (2003). The origins of symbolic racism. Journal of Personality and Social Psychology ,85(2),259.. [DOI] [PubMed] [Google Scholar]
- Sevinc G., Spreng R.N. (2014). Contextual and perceptual brain processes underlying moral cognition: a quantitative meta-analysis of moral reasoning and moral emotions. PloS One, 9(2),e87427.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Tomer R., Berger B.D., Goldsher D., Aharon-Peretz J. (2005). Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cognitive and Behavioral Neurology, 18(1),55–67. [DOI] [PubMed] [Google Scholar]
- Talaska C.A., Fiske S.T., Chaiken S. (2008). Legitimating racial discrimination: emotions, not beliefs, best predict discrimination in a meta-analysis. Social Justice Research, 21, 263–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Humphreys K.L., Shapiro M., Tottenham N. (2013). Amygdala sensitivity to race is not present in childhood but emerges over adolescence. Journal of Cognitive Neuroscience, 25(2),234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawalter S., Todd A.R., Baird A.A., Richeson J.A. (2008). Attending to threat: race-based patterns of selective attention. Journal of Experimental Social Psychology, 44(5),1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urciuoli B. (1993). Representing class: who decides?. Anthropological Quarterly, 203–10. [Google Scholar]
- Volz K.G., Kessler T., von Cramon D.Y. (2009). In-group as part of the self: in-group favoritism is mediated by medial prefrontal cortex activation. Social Neuroscience, 4(3),244–60. [DOI] [PubMed] [Google Scholar]
- Wainryb C. (2006). Moral development in culture: diversity, tolerance, and justice In: Killen M., Smetana J., editors. Handbook of Moral Development (pp. 211–40). USA: Lawrence Erlbaum Associates. [Google Scholar]
- Wang C., Wu B., Liu Y., Wu X., Han S. (2015). Challenging emotional prejudice by changing self-concept: priming independent self-construal reduces racial in-group bias in neural responses to other’s pain. Social Cognitive and Affective Neuroscience, 10(9),1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, B. D. (2002). Deconvolution analysis of fMRI time series data. Milwaukee, WI: Biophysics Research Institute, Medical College of Wisconsin.
- Watson D., Clark L.A. (1999). The Panas-X: Manual for the Positive and Negative Affect Schedule-Expanded Form. Retrieved from http://Ir.Uiowa.Edu/Psychology_Pubs/11
- Weeden K.A., Kim Y.M., Di Carlo M., Grusky D.B. (2007). Social class and earnings inequality. American Behavioral Scientist, 50(5),702–36. [Google Scholar]
- Western M., Wright E.O. (1994). The permeability of class boundaries to intergenerational mobility among men in the United States, Canada, Norway and Sweden. American Sociological Review, 606–29. [Google Scholar]
- Whalen P.J. (1998). Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7, 177–88. [Google Scholar]
- Wheeler M.E., Fiske S.T. (2005). Controlling racial prejudice social-cognitive goals affect amygdala and stereotype activation. Psychological Science, 16(1),56–63. [DOI] [PubMed] [Google Scholar]
- Wilson W.J. (1978). The Declining Significance of Race. Chicago: University of Chicago Press. [Google Scholar]
- Wilson W.J. (1987). The Truly Disadvantaged. Chicago: University of Chicago Press. [Google Scholar]
- Wilson W.J. (2009). More than Just Race: Being Black and Poor in the Inner City. New York: W.W. Norton [Google Scholar]
- Wright E.O. (1997). Class Counts: Comparative Studies in Class Analysis. Cambridge: Cambridge University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.