Abstract
Background
Malaria retinopathy has been proposed as marker of “true” cerebral malaria (CM), ie, coma due to Plasmodium falciparum vs coma due to other causes, with incidental P falciparum parasitemia. Plasma P falciparum histidine-rich protein-2 (PfHRP2) concentrations distinguish retinopathy-positive (RP) from retinopathy-negative (RN) CM but have not been compared between RN CM and other forms of severe malaria or asymptomatic parasitemia (AP).
Methods
We compared plasma PfHRP2 concentrations in 260 children with CM (247 examined for retinopathy), 228 children with severe malarial anemia (SMA), and 30 community children with AP.
Results
Plasmodium falciparum HRP2 concentrations were higher in children with RP CM than RN CM (P = .006), with an area under the receiver operating characteristic curve of 0.61 (95% confidence interval, 0.53–0.68). Plasmodium falciparum HRP2 concentrations and sequestered parasite biomass were higher in RN CM than SMA (both P < .03) or AP (both P < .001).
Conclusions
Plasmodium falciparum HRP2 concentrations are higher in children with RN CM than in children with SMA or AP, suggesting that P falciparum is involved in disease pathogenesis in children with CM. Plasmodium falciparum HRP2 concentrations may provide a more feasible and consistent assessment of the contribution of P falciparum to severe disease than malaria retinopathy.
Keywords: cerebral, malaria, PfHRP2, retinopathy, severe
In 2015, there were an estimated 438000 deaths due to malaria, 90% of which occurred in Africa, and 70% of all deaths occurred in children less than 5 years of age [1, 2]. Mortality due to severe malaria (SM), including cerebral malaria (CM) and SM anemia (SMA), is most often due to Plasmodium falciparum. Cerebral malaria is clinically defined as coma with asexual P falciparum parasitemia and no other known cause of coma. The hallmark in the brain pathology of children who die of CM is the sequestration of parasitized erythrocytes in the cerebral microvasculature, but pathogenesis is also thought to involve inflammation, rosette formation, and dysregulated hemostasis [3, 4].
During the course of P falciparum blood-stage replication, the parasite produces P falciparum histidine-rich protein-2 (PfHRP2) [5]. Most PfHRP2 produced is released into plasma, which allows for estimation of the amount of PfHRP2 produced per parasite per erythrocytic cycle [6]. Plasmodium falciparum HRP2 levels have been used to estimate the total parasite biomass during an infection [7–9]. Studies investigating PfHRP2 levels as a prognostic indicator in SM have shown that PfHRP2 levels (1) can distinguish between uncomplicated malaria (UM) and SM [10–12] and (2) can predict which individuals with UM will go on to develop SM [10]. Plasmodium falciparum HRP2 concentrations can also predict mortality in CM [8, 12, 13]. In addition, recent in vitro and experimental CM studies suggest that PfHRP2 may be directly involved in CM pathogenesis, through activation of the inflammasome and disruption of the blood-brain barrier [14, 15].
Accurate diagnosis of CM, ie, coma due to P falciparum, rather than coma due to other causes with incidental P falciparum parasitemia, remains a challenge in malaria-endemic areas. An autopsy study in Malawian children demonstrated (1) that 23% of children clinically defined as CM had other causes for their coma and death and lacked sequestration of parasitized erythrocytes in the brain microvasculature [16] and (2) that malaria retinopathy at hospitalization was a sensitive and specific indicator of brain parasite sequestration. Therefore, malaria retinopathy has been proposed as marker of “true” CM, as opposed to coma with incidental P falciparum parasitemia.
Total parasite biomass, calculated from plasma PfHRP2 levels, successfully distinguished retinopathy-positive (RP) from retinopathy-negative (RN) CM in a different study in Malawian children [17]. However, there has been no assessment to date of the relative concentrations of PfHRP2 or estimation of sequestered parasite biomass in CM RN children compared with children with other forms of SM or children with asymptomatic parasitemia (AP). If children with RN CM have PfHRP2 levels greater than those with SMA, a known complication of P falciparum, this would suggest that P falciparum is involved in disease pathogenesis in RN CM and that RN CM is therefore also true CM. This is important for research studies and for clinical purposes, because 30%–40% of children with the World Health Organization diagnosis of CM are RN [17, 18]. Inclusion of only RP children in studies of CM, as in some recent studies [19, 20], might exclude substantial numbers of children with true CM, and lack of retinopathy on clinical exam could be misconstrued as evidence that P falciparum is not a cause of coma in the child.
In the present study, we used PfHRP2 testing to compare total, circulating, and sequestered parasite biomass in children with RP and RN CM with children with SMA and with community children (CC) with AP who were from the same neighborhoods or households as children with CM or SMA
METHODS
Study Population, Clinical Assessment, and Ethical Approval
The study was performed at Mulago National Referral and Teaching Hospital in Kampala as previously described [21]. In brief, children with CM, SMA, or CC between 18 months and 12 years of age were enrolled. Cerebral malaria was defined as follows: (1) coma (Blantyre Coma Score ≤2 if <5 years or Glasgow coma score ≤8 if ≥5 years); (2) P falciparum on blood smear; and (3) no other known cause of coma (eg, meningitis, a prolonged postictal state, or hypoglycemia-associated coma reversed by glucose infusion). Severe malaria anemia was defined as presence of P falciparum on blood smear in children with a hemoglobin level ≤5 g/dL. Drs. Susan Lewallen and Simon Harding, ophthalmologists with extensive experience in evaluation of malaria retinopathy in Malawi, trained study medical officers and pediatricians in direct and indirect ophthalmoscopic examination for malaria retinopathy. Medical officers were assessed for accurate evaluation of retinal findings by Drs. Harding in a limited number of children with CM (n = 1 to 3), because children with CM are admitted only intermittently, and for proficiency in indirect ophthalmoscopy through a simulation exercise in which they were required to read letters of newspaper text behind a marble using the indirect ophthalmoscope. After training, medical officers accurately characterized presence of retinopathy in all children with CM examined, when compared to findings by Drs. Lewallen and Harding. Medical officers assessed children for malaria retinopathy, and lead pediatrician investigators provided evaluation of medical officer exams throughout the study.
Children with CM or SMA were managed according to the Ugandan Ministry of Health treatment guidelines current at the time of the study. These included (1) intravenous quinine treatment followed by oral quinine for SM while admitted and (2) artemisinin combination therapy for outpatient follow-up therapy. Healthy CC were recruited from the extended family or household compound area of children with CM or SMA. Peripheral blood smears were prepared for all CC, and CC with P falciparum infection were treated with artemether-lumefantrine. Microscopy for peripheral parasitemia and laboratory testing for complete blood and platelet counts, and glucose and human immunodeficiency virus testing were performed as previously described [21].
Written informed consent was obtained from parents or guardians of study participants. The studies were reviewed and approved by the Ugandan National Council for Science and Technology, the Makerere University School of Medicine Research and Ethics Committee, and the University of Minnesota Institutional Review Board.
Plasmodium falciparum Histidine-Rich Protein-2 Testing
Plasma PfHRP2 levels were quantified using the commercially available Malaria Ag CELISA kit (Cellabs, Brookvale, Australia). Plasma was diluted 1:2400. Samples with results above or below the range of the standard curve were retested at a dilution of 1:24000 or 1:200, respectively. To assess intra-assay reproducibility, 10% of samples were randomly selected from each assay plate to retest on subsequent plates. The coefficient of variance for these samples was 22%. For analysis, PfHRP2 concentrations below the lowest detectable standard were transformed to lowest detectable standard value (4.8 ng/mL).
Estimation of Parasite Biomass
Parasite biomass (total, circulating, sequestered) was estimated using previously published calculations [7, 8]. In brief, the calculation for total and circulating biomass is Ptot = 7.3 × PfHRP2 [g/L] × (1-hematocrit) × body weight [kg] × 1013; Pcirc = parasites/μL × 106 × hematocrit × blood volume [estimated at 0.08 L/kg × body weight]. Sequestered biomass is calculated as Pseq = Ptot − Pcirc.
Statistical Analyses
All statistical analyses were completed using STATA SE version 12.0 (Stata Corporation, College Station, TX). Study participant characteristics and PfHRP2 concentrations were compared using the Wilcoxon rank-sum, Spearman’s rank correlation, and Pearson’s χ2 tests. Differences were considered significant at P < .05. Receiver operating characteristic (ROC) analyses were used to determine whether PfHRP2 could distinguish between disease groups.
RESULTS
Clinical and Laboratory Characteristics of Study Children
A total of 267 children with CM, 232 children with SMA, and 214 CC were enrolled in the study, and 30 of the CC had AP. Two hundred sixty children with CM, 228 with SMA, and all 30 AP had sufficient plasma for PfHRP2 testing. A higher proportion of children with SM compared with AP were male, and, as expected, children with SM had lower hemoglobin levels and lower platelet counts than AP (Table 1).
Table 1.
Clinical and Laboratory Characteristics of Children With Cerebral Malaria, Severe Malarial Anemia, and Community Children With Asymptomatic Parasitemia
| Characteristic | CMa | N | SMAa | N | APa | N | P, CM SMAb | P, CM APb | P, SMA APb |
|---|---|---|---|---|---|---|---|---|---|
| Sex, n (%) female | 106 (40.8) | 260 | 89 (39.0) | 228 | 106 (53.3) | 30 | .70 | .54 | .42 |
| Age, years | 3.99 (2.0) | 260 | 3.37 (1.7) | 228 | 3.96 (1.5) | 30 | <.001 | .95 | .072 |
| Weight, kg | 14.4 (4.4) | 260 | 12.8 (3.7) | 228 | 15.0 (3.9) | 30 | <.001 | .52 | .003 |
| Hemoglobin, g/dL | 7.0 (2.3) | 260 | 3.8 (0.9) | 228 | 11.3 (1.8) | 27 | <.001 | <.001 | <.001 |
| Platelets, 106/mL | 83 (82) | 255 | 184 (138) | 226 | 250 (127) | 27 | <.001 | <.001 | .019 |
| Parasite density, parasites/μL, median (interquartile range) | 48 080 (11 120–282 400) | 260 | 36 150 (10 510–144 670) | 228 | 1290 (580–8360) | 30 | .03 | <.001 | <.001 |
| HIV positive, n (%) | 5 (2.1) | 241 | 6 (2.7) | 225 | 0 (0) | 30 | .67 | .43 | .37 |
| Mortality, n (%) | 31 (11.9) | 260 | 1 (0.4) | 228 | 0 (0) | 30 | <.001 | .05 | .72 |
Values in bold indicate P <.05.
Abbreviations: AP, asymptomatic parasitemia; CM, cerebral malaria; HIV, human immunodeficiency virus; SMA, severe malarial anemia.
aData are mean (standard deviation) unless otherwise indicated.
bCompared by χ2 test (sex, HIV status, and mortality), Student’s t test (age, weight, hemoglobin, platelet count), or Wilcoxon rank-sum test (parasite density).
Malaria Retinopathy in Children With Cerebral Malaria
Two hundred forty-seven children with CM had a retinal examination, and 161 of these children (65.2%) had 1 or more features of malarial retinopathy (retinal hemorrhages, macular whitening, peripheral whitening, or vessel changes). Some of the children did not have fundoscopy due to death before examination, inadequate pupil dilation, or awaking from coma before the exam could be performed. Among the 161 children with CM who had retinopathy, the most common findings were retinal hemorrhages (n = 136, 84.5%), macular whitening (n = 83, 51.6%), peripheral whitening (n = 57, 35.4%), and vessel changes (n = 57, 35.4%). Among children with CM, RP children had significantly lower hemoglobin levels and lower platelet counts than RN children, but no other demographic or clinical characteristics differed by retinopathy findings (Table 2). Demographic and clinical differences seen between children with SMA and CM were seen regardless of whether the children with CM were RP or RN (Table 2).
Table 2.
Clinical and Laboratory Characteristics of Children With Retinopathy-Positive Cerebral Malaria, Retinopathy-Negative Cerebral Malaria, and Severe Malarial Anemia
| Characteristic | RPa | N | RNa | N | SMAa | N | P, RP RNb | P, RP SMAb | P, RN SMAb |
|---|---|---|---|---|---|---|---|---|---|
| Sex, n (%) female | 69 (42.9) | 161 | 34 (39.5) | 86 | 89 (39.0) | 228 | .61 | .45 | .94 |
| Age, years | 3.84 (2.0) | 161 | 4.12 (1.9) | 86 | 3.37 (1.7) | 228 | .28 | .01 | <.001 |
| Weight, kg | 14.1 (4.5) | 161 | 14.8 (4.4) | 86 | 12.8 (3.7) | 228 | .23 | .003 | <.001 |
| Hemoglobin, g/dL | 6.5 (2.2) | 161 | 7.7 (2.2) | 86 | 3.8 (0.9) | 228 | <.001 | <.001 | <.001 |
| Platelets, 106/mL | 74 (52) | 157 | 95 (107) | 86 | 184 (138) | 226 | 0.04 | <.001 | <.001 |
| Parasite density, parasites/μL, median (interquartile range) | 47 880 (9920–294 500) | 161 | 49 765 (15 480–273 100) | 86 | 36 150 (10 510–144 670) | 228 | .46 | .10 | .03 |
| HIV positive, n (%) | 2 (1.3) | 149 | 3 (3.7) | 81 | 6 (2.7) | 225 | .24 | .39 | .64 |
| Mortality, n (%) | 23 (14.3) | 161 | 6 (7.0) | 86 | 1 (0.4) | .09 | <.001 | <.001 |
Values in bold indicate P <.05.
Abbreviations: HIV, human immunodeficiency virus; RN, retinopathy negative; RP, retinopathy positive; SMA, severe malarial anemia.
aData are mean (standard deviation) unless otherwise indicated.
bCompared by χ2 test (sex, HIV status, and mortality), Student’s t test (age, weight, hemoglobin, platelets), or Wilcoxon rank-sum test (parasite density).
Plasmodium falciparum Histidine-Rich Protein-2 Levels in Children With Severe Malarial Anemia and in Children With Cerebral Malaria With and Without Retinopathy
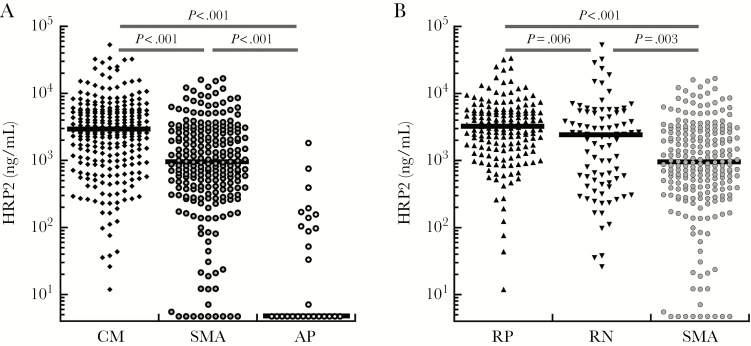
Plasmodium falciparum HRP2 levels were highest in children with CM but higher in children with SMA than in AP (Figure 1A). Among children with CM, RP children had significantly higher levels of PfHRP2 than RN children and children with SMA (Figure 1B). In addition, PfHRP2 levels were significantly higher in RN CM children than children with SMA (Figure 1B and Table 4). Among the 30 CC with AP, PfHRP2 levels were significantly lower (median, 4.8 ng/mL; interquartile range [IQR], 4.8–106) than in children with RP CM (median, 3163 ng/mL; IQR, 1538–5405; P < .001), RN CM (median, 2365 ng/mL; IQR, 516–5176; P < .001) or SMA (median, 929 ng/mL; IQR, 379–2735; P < .001).
Figure 1.
Plasmodium falciparum histidine-rich protein-2 (PfHRP2) levels in children with severe malaria. Black horizontal bars within the scatter plots indicate the group median, and black horizontal bars above the scatter plots show the group comparisons and associated P values. (A) Plasma HRP2 levels in children with cerebral malaria (CM), children with severe malarial anemia (SMA), and community children with asymptomatic parasitemia (AP). (B) Plasma HRP2 levels in children with SMA or with CM and retinopathy (RP) or no retinopathy (RN).
Table 4.
Parasite Biomass ×1010 in Children With Severe Malaria
| Parasite Biomass | RP (N = 161)a | RN (N = 86)a | SMA (N = 228)a | P, RP RNb | P, RP SMAb | P, RN SMAb |
|---|---|---|---|---|---|---|
| PfHRP2, ng/mL | 3163 (1538–5405) | 2365 (516–5176) | 929 (379–2735) | .006 | <.001 | .003 |
| Total | 266 (123–448) | 176 (37.7–357) | 78.5 (29.2–191) | .007 | <.001 | .002 |
| Circulating | 4.9 (1.1–32.4) | 5.7 (1.7–23.6) | 3.7 (1.0–13.2) | .36 | .04 | .007 |
| Sequestered | 237 (103–416) | 128 (25.4–351) | 64.7 (21.4–178) | .005 | <.001 | .02 |
Values in bold indicate P <.05.
Abbreviations: PfHRP2, Plasmodium falciparum histidine-rich protein-2; RN, retinopathy negative cerebral malaria; RP, retinopathy positive cerebral malaria; SMA, severe malarial anemia.
aData are median (interquartile range).
bCompared by Wilcoxon rank-sum test.
Utility of Plasmodium falciparum Histidine-Rich Protein-2 Levels to Distinguish Between Retinopathy-Positive and Retinopathy-Negative Cerebral Malaria and Asymptomatic Parasitemia
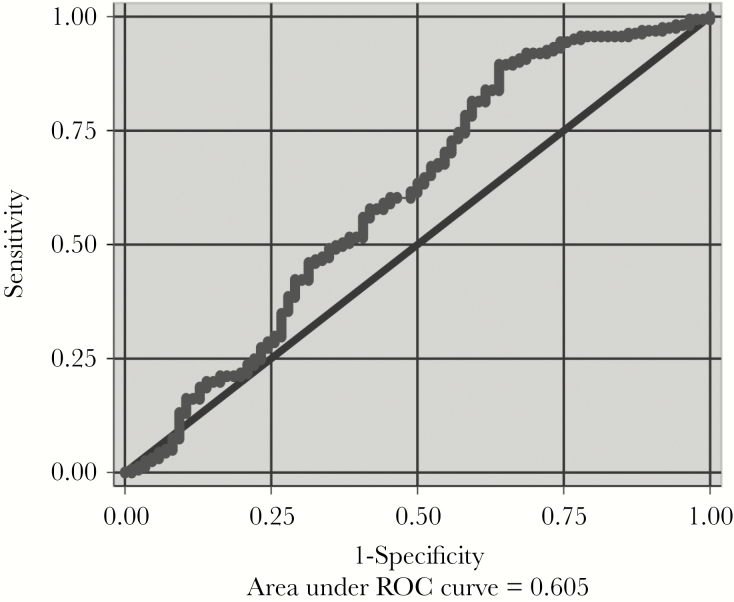
Receiver operating characteristic analyses revealed that the area under the ROC curve (AUROC) of PfHRP2 for RP vs RN CM was 0.61 (95% confidence interval [CI], 0.53–0.68) (Figure 2). A cutoff of 1392 ng/mL maximized sensitivity and specificity for RP CM compared with RN CM (sensitivity 78.3% [95% CI, 71.1%–84.4%], specificity 41.9% [95% CI, 31.3%–60.0%], negative predictive value (NPV) 50.7% [95% CI, 38.6%–62.8%], and positive predictive value (PPV) 71.6% [95% CI, 64.3%–78.1%]). Using the PfHRP2 cutoff value of 1700 ng/mL from Seydel et al [17] yielded sensitivity for RP vs RN CM of 72.7% (95% CI, 65.1%–79.4%), specificity 44.2% (95% CI, 33.5%–55.3%), NPV 46.3% (95% CI, 35.3%–57.7%), and PPV 70.9% (95% CI, 63.3%–77.7%).
Figure 2.
Receiver operating characteristic (ROC) analyses of Plasmodium falciparum histidine-rich protein-2 (PfHRP2) concentrations in children with cerebral malaria (CM) and with retinopathy (RP) or without retinopathy (RN). The area under the ROC indicates the accuracy of HRP2 concentration in discriminating between RP and RN CM.
Plasmodium falciparum HRP2 concentration distinguished well between CM and AP (AUROC 0.97; 95% CI, 0.94–1.0), including between RP CM and AP (AUROC 0.98; 95% CI, 0.96–1.0) and between RN CM and AP (AUROC 0.95; 95% CI, 0.91–0.99). A PfHRP2 cutoff value of 232 ng/mL provided a sensitivity of >90% for RP and RN CM compared with AP, with optimal specificity: RP CM, sensitivity 96.9% (95% CI, 92.9%–99.0%), specificity 90.0% (95% CI, 73.5%–97.9%), NPV 84.4% (95% CI, 67.2%–94.7%), PPV 98.1% (95% CI, 94.6%–99.6%); RN CM, sensitivity 91.9% (95% CI, 84.0%–96.7%), specificity 90.0 % (95% CI, 73.5% – 97.9%), NPV 79.4% (95% CI, 62.1%–91.3%), and PPV 96.3% (95% CI, 89.7%–99.2%).
Plasmodium falciparum Histidine-Rich Protein-2 Levels and Mortality
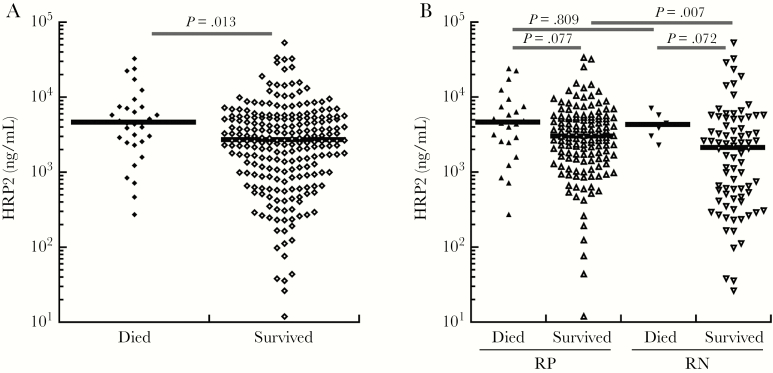
Among children with CM, PfHRP2 levels were significantly higher in children who died than in survivors (survived [n = 229; median, 2654; IQR, 996–5362] vs died [n = 31; median, 4514; IQR, 2489–7469], P = .013) (Figure 3A). A nonsignificant trend toward increased mortality was seen in RP (23 of 161, 14.3%) vs RN (6 of 86, 7.0%) children. The PfHRP2 levels in RP and RN children with fatal CM did not differ (P = .81); however, among survivors of CM, PfHRP2 levels were higher in RP children than RN children (P = .007) (Figure 3B).
Figure 3.
Plasmodium falciparum histidine-rich protein-2 (PfHRP2) levels stratified by outcome in children with cerebral malaria (CM). (A) Plasma HRP2 levels in children with CM that either died or survived. Black horizontal bars within the scatter plots indicate the group median, and black horizontal bars above the scatter plots show the group comparisons and associated P values. (B) Plasma HRP2 levels in children with CM that either died or survived additionally stratified by those with retinopathy (RP) or without retinopathy (RN).
Parasite Biomass
Total, circulating, and sequestered parasite biomass all differed significantly between CM, SMA, and CC groups, with levels highest in CM, followed by SMA, followed by CC (Table 3). Total and sequestered, but not circulating, parasite biomass were higher in RP than RN CM, and total, sequestered, and circulating parasite biomass were all higher in RP or RN CM than in SMA (Table 4). Finally, total, sequestered, and circulating parasite biomass were higher in RN CM than in AP (P < .001 for all) (Tables 3 and 4).
Table 3.
Parasite Biomass ×1010
| Parasite Biomass | CM (N = 260)a | SMA (N = 228)a | AP (N = 30)a | P, CM SMAb | P, CM APb | P, SMA APb |
|---|---|---|---|---|---|---|
| PfHRP2, ng/mL | 2864 (1092–5537) | 929 (379–2735) | 4.8 (4.8–105.6) | <.001 | <.001 | <.001 |
| Total | 223 (87.8–450) | 78.5 (29.2–191) | 0.4 (0.3–9.5) | <.001 | <.001 | <.001 |
| Circulating | 5.2 (1.2–28.6) | 3.7 (1.0–13.2) | 0.1 (0.1–1.0) | .006 | <.001 | <.001 |
| Sequestered | 188 (70.4–414) | 64.7 (21.4–178) | 0.3 (0.1–7.4) | <.001 | <.001 | <.001 |
Values in bold indicate P <.05.
Abbreviations: AP, asymptomatic parasitemia in community children; CM, cerebral malaria; PfHRP2, Plasmodium falciparum histidine-rich protein-2; SMA, severe malarial anemia.
aData are median (interquartile range). N = 27 for AP total and sequestered biomass.
bCompared by Wilcoxon rank-sum test.
DISCUSSION
In the present study, although plasma PfHRP2 concentrations distinguished between RP and RN CM, plasma PfHRP2 concentrations and total and sequestered parasite biomass in children with RN CM were significantly higher than in children with SMA, another form of SM, and far higher than in children with asymptomatic P falciparum parasitemia. Because PfHRP2 concentrations and sequestered parasite biomass are markers of disease severity in malaria, the study findings support a role for P falciparum in the pathogenesis of RN CM.
An important limitation of the present study and any study of malaria retinopathy is the expertise of those doing the testing. The level of expertise required for indirect and direct ophthalmoscopy is available in few African or Asian medical centers. In the present study, medical officers trained by expert ophthalmologists with expertise in assessment of malaria retinopathy did all primary assessments of malaria retinopathy. The significant difference in PfHRP2 levels between RP and RN children with CM, together with the similar percentage of RP children among all children with CM in the present study and in a Malawian study on PfHRP2 levels in RP and RN CM [17] (66.7% and 70.3%, respectively) suggest a reliable assessment of retinopathy in our study.
In the Malawi study, PfHRP2 levels distinguished better between RP and RN CM than in the present study (AUROC = 0.90 vs 0.63, respectively). In addition, in the present study, PfHRP2 levels did not differ between RP and RN children who died, whereas they did differ in children who survived. In the Malawi studies, exams were done by ophthalmologists as opposed to medical officers, and therefore they were likely to have greater sensitivity and specificity for detection of malaria retinopathy. However, because our medical officers were trained by the expert ophthalmologists who conducted the Malawi studies, we believe that our study assessment of malaria retinopathy is an optimal “real-world” assessment and valid for testing the question of whether RN CM, as assessed in the field, reflects coma due to causes other than P falciparum. In addition, PfHRP2 levels distinguished well between RN CM and AP in this community, suggesting that children with RN CM are unlikely to have incidental parasitemia. The overlap of PfHRP2 levels in children with RN CM and children with SMA further argues that P falciparum is involved in this disease process as it is in SMA. Given the lower mortality in the RN than RP CM group (14.3% vs 7.0%), it is possible that RN CM represented a less severe form of CM, consistent with the lower sequestered parasite biomass seen in RN CM. This could have led to a lack of malaria retinopathy or less easily detectable malaria retinopathy. It is also possible that children with RN CM do have P falciparum as the cause of coma but are diagnosed earlier and so have not yet had time to develop retinopathy.
A study in Gambian children showed that, as in this study, children with SMA have a high sequestered parasite biomass [9], whereas a study in Malawi showed that 53% of children with SMA had malaria retinopathy [22], demonstrating that retinopathy (and presumably associated brain-level sequestration) are not exclusive to CM and therefore not a sufficient factor for development of CM. Taken together, the findings of the present study and previous studies suggest that sequestration in the postcapillary venules of the brain is an important component of the disease process in most cases of CM but that sequestration in the brain, as reflected by malaria retinopathy, does not inevitably lead to coma. The study findings do not necessarily contradict the findings of the landmark study by Taylor et al [16], showing that parasites were not present in children with RN CM on autopsy, because that study assessed only children who died of CM. It is possible that survivors of CM differ from those who die of CM, and that those who die have more advanced disease in which retinopathy is almost uniformly present, whereas some who survive may do so without development of clinically discernible retinopathy.
Assessment of PfHRP2 levels can be affected by several factors, including variations in parasite genotype or gene expression [23–26], prior malaria exposure [27], type of detection assay used, and stage of parasite at time of assessment. None of these are likely to have had a major effect on the major study finding of high PfHRP2 levels in RN CM, because the kit for antibody testing was used in the prior large studies of PfHRP2 levels in SM [7–9, 17], and all of these factors would generally lead to lack of detection of PfHRP2, whereas our findings demonstrated high levels of PfHRP2 in children with RN CM. Optimal cutoffs for prediction of severe disease, mortality, and malaria-attributable disease will require additional studies. The coefficient of variance for PfHRP2 testing in the present study was 22%, which could have contributed to a degree of variability in results.
Plasmodium falciparum HRP2 may also play a role in disease pathogenesis. Pathways through which PfHRP2 could lead to severe disease in malaria include mediation of the formation of hemozoin, detoxifying heme for the parasite, and binding of heparin and other glycosaminoglycans [28], leading to microvascular sequestration. Plasmodium falciparum HRP2 may also have an immunomodulatory role in SM, because exposure of T cells to PfHRP2 can inhibit the expression of CD69, decrease interferon-γ secretion, and decrease lymphocyte proliferation, potentially dampening initial protective immune responses against the parasite [29]. Recently, Pal et al [15] demonstrated (1) that disruption of the human microvascular endothelial barrier depends on expression of PfHRP2 and (2) that recombinant or purified HRP2 recapitulated these effects through activation of the inflammasome, which decreased integrity of tight junctions and increased endothelial permeability. They subsequently showed (1) that intravenous administration of PfHRP2 induced blood-brain barrier leakage in uninfected mice and (2) that PfHRP2 infusion in Plasmodium berghei-infected mice increased early mortality from experimental CM [14]. These findings provide the clearest evidence to date that PfHRP2 may be involved in disease pathogenesis in CM. The increasing PfHRP2 concentrations seen with increasing disease severity (SMA, RN CM, RP CM) in this study are consistent with this idea.
CONCLUSIONS
In summary, the present study shows that children with RN CM have substantial sequestered P falciparum biomass, which suggests that P falciparum is a contributor to disease pathogenesis in children with RN CM, as it is in RP CM. It is possible that some children with RN CM in this study were misclassified and malaria retinopathy, but the findings likely reflect a close to optimal capture of malaria retinopathy within most clinical settings in sub-Saharan Africa. The study data add to the complexity of defining true CM but support the idea that measurement of PfHRP2 levels, for which low-cost, low-training, point-of-care devices should be feasible, are useful in distinguishing whether severe disease is due to P falciparum malaria or other causes. We are pursuing investigations of factors involved in endothelial activation, inflammation, and hemostasis in children with RN and RP CM, to better determine whether the pathophysiology of RN CM is distinguishable from RP CM. Additional studies, ideally with photographic evidence of retinopathy, are needed to determine whether the present study findings are more broadly applicable to other malaria-endemic areas.
Acknowledgments
We thank the study participants and their guardians for their participation in the study. We also thank the study team at Makerere University including the following: medical officers (Drs. Peter Katumba, Ahmmed Ddungu, and Victor Tumukunde), nurses, data entry personnel, and Karen Hamre for statistical assistance.
Financial support. This work was supported by grants from the National Institute of Neurological Disorders and Stroke and the Fogarty International Center (R01NS055349 and D43NS078280; to C. C. J.) and from the University of Minnesota Undergraduate Research Opportunities Program (to A. W.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World Malaria Report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 2. Murray CJ, Rosenfeld LC, Lim SS et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 2012; 379:413–31. [DOI] [PubMed] [Google Scholar]
- 3. Haldar K, Murphy SC, Milner DA, Taylor TE. Malaria: mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu Rev Pathol 2007; 2:217–49. [DOI] [PubMed] [Google Scholar]
- 4. van der Heyde HC, Nolan J, Combes V et al. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol 2006; 22:503–8. [DOI] [PubMed] [Google Scholar]
- 5. Howard RJ, Uni S, Aikawa M et al. Secretion of a malarial histidine-rich protein (Pf HRP II) from Plasmodium falciparum-infected erythrocytes. J Cell Biol 1986; 103:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desakorn V, Dondorp AM, Silamut K et al. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans R Soc Trop Med Hyg 2005; 99:517–24. [DOI] [PubMed] [Google Scholar]
- 7. Dondorp AM, Desakorn V, Pongtavornpinyo W et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med 2005; 2:e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hendriksen IC, Mwanga-Amumpaire J, von Seidlein L et al. Diagnosing severe falciparum malaria in parasitaemic African children: a prospective evaluation of plasma PfHRP2 measurement. PLoS Med 2012; 9:e1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cunnington AJ, Bretscher MT, Nogaro SI et al. Comparison of parasite sequestration in uncomplicated and severe childhood Plasmodium falciparum malaria. J Infect 2013; 67:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fox LL, Taylor TE, Pensulo P et al. Histidine-rich protein 2 plasma levels predict progression to cerebral malaria in Malawian children with Plasmodium falciparum infection. J Infect Dis 2013; 208:500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manning L, Laman M, Stanisic D et al. Plasma Plasmodium falciparum histidine-rich protein-2 concentrations do not reflect severity of malaria in Papua New Guinean children. Clin Infect Dis 2011; 52:440–6. [DOI] [PubMed] [Google Scholar]
- 12. Hendriksen IC, White LJ, Veenemans J et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis 2013; 207:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanson J, Lam SW, Mahanta KC et al. Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis 2012; 206:571–9. [DOI] [PubMed] [Google Scholar]
- 14. Pal P, Balaban AE, Diamond MS et al. Plasmodium falciparum histidine-rich protein II causes vascular leakage and exacerbates experimental cerebral malaria in mice. PLoS One 2017; 12:e0177142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pal P, Daniels BP, Oskman A et al. Plasmodium falciparum histidine-rich protein II compromises brain endothelial barriers and may promote cerebral malaria pathogenesis. MBio 2016; 7:e00617–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor TE, Fu WJ, Carr RA et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 2004; 10:143–5. [DOI] [PubMed] [Google Scholar]
- 17. Seydel KB, Fox LL, Glover SJ et al. Plasma concentrations of parasite histidine-rich protein 2 distinguish between retinopathy-positive and retinopathy-negative cerebral malaria in Malawian children. J Infect Dis 2012; 206:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Villaverde C, Namazzi R, Shabani E et al. Clinical comparison of retinopathy-positive and retinopathy-negative cerebral malaria. Am J Trop Med Hyg 2017; 96:1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Birbeck GL, Molyneux ME, Kaplan PW et al. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol 2010; 9:1173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boivin MJ, Gladstone MJ, Vokhiwa M et al. Developmental outcomes in Malawian children with retinopathy-positive cerebral malaria. Trop Med Int Health 2011; 16:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bangirana P, Opoka RO, Boivin MJ et al. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis 2014; 59:336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beare NA, Southern C, Chalira C et al. Prognostic significance and course of retinopathy in children with severe malaria. Arch Ophthalmol 2004; 122:1141–7. [DOI] [PubMed] [Google Scholar]
- 23. Baker J, Gatton ML, Peters J et al. Transcription and expression of Plasmodium falciparum histidine-rich proteins in different stages and strains: implications for rapid diagnostic tests. PLoS One 2011; 6:e22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baker J, Ho MF, Pelecanos A et al. Global sequence variation in the histidine-rich proteins 2 and 3 of Plasmodium falciparum: implications for the performance of malaria rapid diagnostic tests. Malar J 2010; 9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramutton T, Hendriksen IC, Mwanga-Amumpaire J et al. Sequence variation does not confound the measurement of plasma PfHRP2 concentration in African children presenting with severe malaria. Malar J 2012; 11:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scherf A, Mattei D. Cloning and characterization of chromosome breakpoints of Plasmodium falciparum: breakage and new telomere formation occurs frequently and randomly in subtelomeric genes. Nucleic Acids Res 1992; 20:1491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biswas S, Tomar D, Rao DN. Investigation of the kinetics of histidine-rich protein 2 and of the antibody responses to this antigen, in a group of malaria patients from India. Ann Trop Med Parasitol 2005; 99:553–62. [DOI] [PubMed] [Google Scholar]
- 28. Ndonwi M, Burlingame OO, Miller AS et al. Inhibition of antithrombin by Plasmodium falciparum histidine-rich protein II. Blood 2011; 117:6347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Das P, Grewal JS, Chauhan VS. Interaction of Plasmodium falciparum histidine-rich protein II with human lymphocytes leads to suppression of proliferation, IFN-gamma release, and CD69 expression. Parasitol Res 2006; 100:39–50. [DOI] [PubMed] [Google Scholar]