FIG 3 .
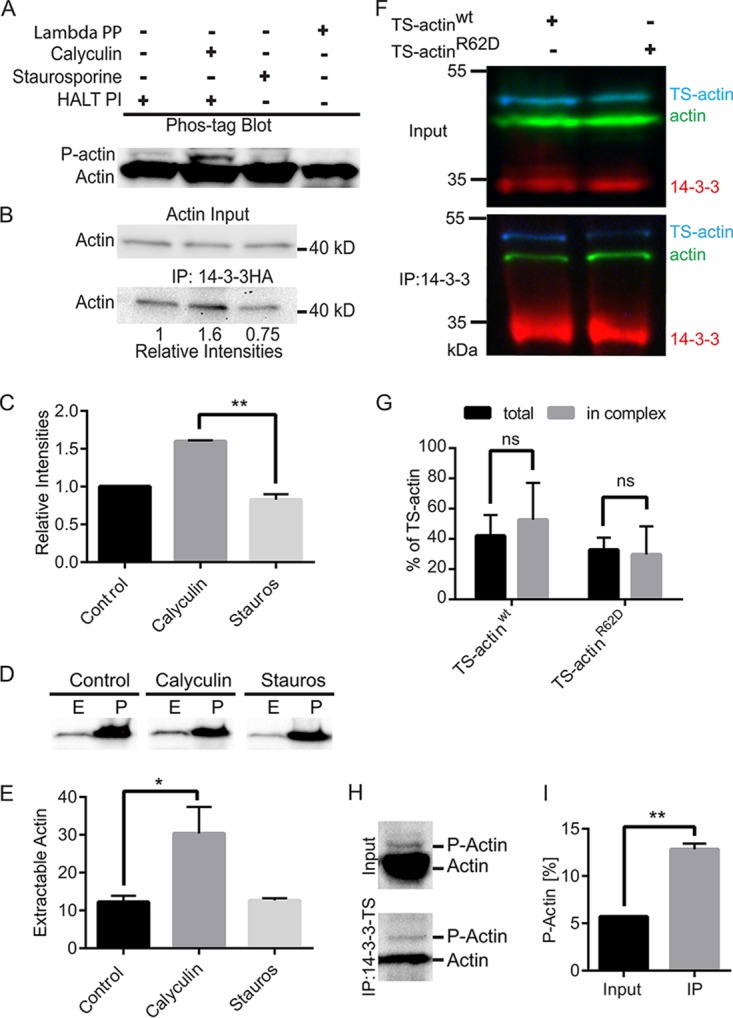
14-3-3–actin complex formation is phospho-dependent. (A) Immunoblot after Phos-tag phosphate-affinity electrophoresis. Cells were pretreated with DMSO or inhibitors and then HALT phosphatase inhibitor (HALT PI) was added at lysis to preserve the phosphorylation state. Calyculin A treatment increased phosphorylated-actin (P-actin) levels, and the kinase inhibitor staurosporine reduced P-actin. Phospho-isoforms were removed after lambda protein phosphatase (PP) treatment. (B) Immunoprecipitation of Gl-14-3-3–HA after calyculin A treatment led to increased actin interaction, while staurosporine treatment reduced the association of actin with Gl-14-3-3–HA. (C) Mean values of three independent experiments. Error bars represent SD. **, P < 0.01. (D) Detergent-extractable actin is increased by calyculin A treatment. E, extracted, predominantly G-actin; P, cell pellet/nonextracted, predominantly F-actin. (E) Plots are mean percentages of extractable actin from three independent experiments. Error bars represent SD. *, P < 0.05. (F) Pulldown of 14-3-3 in cells expressing wild-type TS-actin or the polymerization-defective R62D mutant. (G) Graph showing binding of wild-type TS-actin compared to the R62D polymerization-defective mutant in three independent experiments. ns, not statistically significant. (H) Compared with input, eluted protein from 14-3-3–TS pulldown shows enrichment of P-actin. (I) Quantification of three independent experiments. **, P < 0.01.
