FIG 7 .
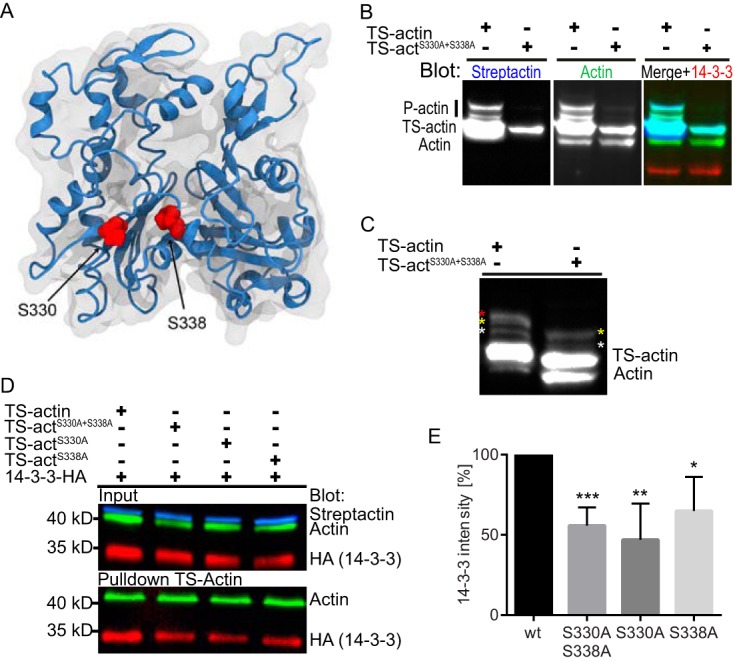
S330 and S338 of Gl-actin contribute to 14-3-3 complex formation. (A) Model of Gl-actin showing the positions of S330 and S338 in an actin monomer. (B) Multiplexed immunoblot of total Giardia extracts after Phos-tag phosphate-affinity electrophoresis comparing phosphorylation of TS-actin and TS-actinS330A S338A; anti-Gl-actin (green), StrepTactin-HRP (blue), and anti-HA (red). Note equal loading as indicated by 14-3-3 levels. (C) Samples from panel B were overloaded and probed with anti-Gl-actin antibody. Colored asterisks mark specific P-actin bands: note that only two bands are visible in the TS-actinS330A S338A double mutant. (D) Affinity pulldown of TS-actin variants blotted for Gl-actin and 14-3-3–HA. (E) Quantification of three independent affinity pulldown experiments shows S330 and S338 contribute to 14-3-3 association. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
