Figure 1.
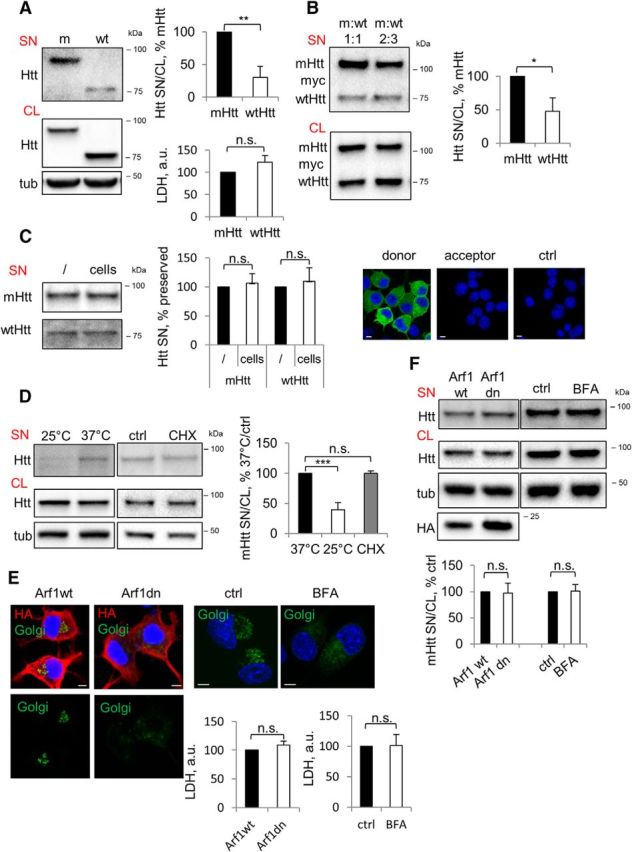
Preferential secretion of mHtt via unconventional secretory pathway. A, mHtt is secreted preferentially compared with wtHtt. Left, Neuro2A-mHtt cells were incubated for the final 16 h in OptiMEM of total 48 h after plating. Equal volumes of the concentrated media (SN) and cell lysates (CL) were analyzed by Western blotting using anti-Htt antibody. Tubulin was used as a loading control. Top right, Ratio of Htt in the media and cell lysates expressed as a percentage of mHtt. n = 3, p = 0.0021. Bottom right, LDH assay was performed on the media before concentrating. n = 3, p = 0.1601. B, mHtt is secreted preferentially from cells coexpressing mHtt and wtHtt. Left, Naive Neuro2A cells were cotransfected with 590aa Htt/myc/His/97Q (mHtt) and 25Q (wtHtt) at the ratios 1:1 (left lanes) and 2:3 (right lanes). Cells were incubated for the final 16 h of total 48 h after transfection in OptiMEM. Concentrated media (SN) and cell lysates (CL) were analyzed by Western blotting using anti-myc antibody. Right, Ratio of Htt in the media and cell lysates expressed as a percentage of mHtt. n = 3, p = 0.0114. C, Secreted Htt is not depleted from the media through endocytosis. Left, Media conditioned overnight on Neuro2A stably expressing mHtt or wtHtt was collected and further incubated in empty wells (/) or with naive Neuro2A cells (cells) for 4 h. Media was then concentrated and analyzed by Western blotting using anti-Htt antibody. Center, Htt in the media incubated with cells expressed as a percentage of Htt in the media incubated in empty wells. n = 3; p = 0.5230 and 0.5183. Right, Media conditioned on Neuro2A cells transiently expressing mHtt-GFP (donor cells) was transferred on naive Neuro2A (acceptor cells) and incubated for 4 h in the presence of 200 nm bafilomycin A1 to prevent degradation of putatively endocytosed Htt. Naive Neuro2A not exposed to Htt-conditioned media was used as a negative control (ctrl). Cells were then fixed, mounted, and analyzed by confocal microscopy. Nuclei were visualized using DAPI staining. n = 3. D, mHtt secretion is temperature dependent. Neuro2A-mHtt cells were incubated at 25°C or 37°C on the benchtop for 4 h in OptiMEM (left) and at 37°C with 10 μg/ml cycloheximide (CHX) or DMSO (ctrl) (center). Concentrated media and cell lysates were analyzed by Western blotting using anti-Htt antibody. Tubulin was used as a loading control. Right, Ratio of mHtt in the media and cell lysates expressed as a percentage of 37°C control. n = 3, p = 0.0009 and 0.957. E, Genetic and pharmacological disruption of the constitutive secretory pathway leads to collapse of the Golgi complex and is nontoxic. Left, Neuro2A-mHtt cells were transfected with HA-tagged Arf1wt or Arf1T31N (Arf1dn) and incubated for the last 16 of total 24 h after transfection in OptiMEM. Right, Cells were treated with DMSO (ctrl) or 5 μg/ml brefeldin A (BFA) in preconditioned OptiMEM for 1 h. Cells were then transduced with CellLight Golgi-GFP for the last 16 h of incubation and analyzed by confocal microscopy upon immunolabeling with anti-HA antibody (left) or without additional staining (right). Nuclei were visualized using DAPI staining. Scale bar, 10 μm. Bottom, LDH assay was performed on 30 μl of the media before concentrating. n = 3, p = 0.2189 and 0.9424. F, mHtt secretion does not follow a constitutive secretion pathway. Top, Cells were treated as in E. Concentrated media and the cell lysates were analyzed by Western blotting using anti-Htt antibody. Tubulin was used as a loading control. Bottom, Ratio of mHtt in the media and cell lysates expressed as a percentage of control. n = 3, p = 0.91 and 0.89. Error bars indicate SD. *p < 0.05; **p < 0.01; ***p < 0.001; n.s., Not significant.
