Abstract
Background
Retinopathy is increasingly recognized in prediabetic populations, and may herald increased risk of metabolic worsening. The Early Diabetes Intervention Program (EDIP) evaluated worsening of glycemia in screen-detected Type 2 diabetes, following participants for up to 5 years. Here we have evaluated whether the presence of retinopathy at the time of detection of diabetes was associated with accelerated progression of glycemia.
Methods
We prospectively studied 194 participants from EDIP with available baseline retinal photographs. Retinopathy was determined at baseline using 7-field fundus photography and defined as an Early Treatment of Diabetic Retinopathy Study Scale grading score of ≥20.
Results
At baseline, 12% of participants had classical retinal lesions indicating retinopathy. In univariate Cox proportional hazard analysis, the presence of retinopathy at baseline was associated with a doubled risk of progression of fasting plasma glucose (HR 2.02; 95% CI 1.05–3.89). The retinopathy effect was robust to individual adjustment for age and glucose, the most potent determinants of progression in EDIP.
Conclusion
Retinopathy was associated with increased risk of progression of fasting plasma glucose among adults with screen-detected, early diabetes. Early detection of retinopathy may help individualize more aggressive therapy to prevent progressive metabolic worsening in early diabetes.
Keywords: retinopathy, early diabetes, diabetes, microvascular disease
Introduction
Microvascular disease of the retina is a common complication of diabetes, and in populations with Type 1 and Type 2 diabetes the prevalence and progression of retinopathy are clearly related to hyperglycemia. Retinal microvascular lesions typical of diabetic eye disease are also detectable in people without diabetes (Klein, 1992; Leibowitz et al., 1980; Wong, Klein, Sharrett, et al., 2003; Yu, Mitchell, Berry, Li, & Wang, 1998), and in these populations the clinical significance of these retinal lesions is less clear (Wong, Klein, Klein, et al., 2001). While these lesions may not be related to glycemia (Diabetes Prevention Program Research Group, 2007) they have been found to be associated with older age and systemic hypertension (Klein, Klein, Moss, & Wang, 1994; Sharp et al., 1995; Svardsudd, Wedel, Aurell, & Tibblin, 1978; Yu et al., 1998), suggesting association with cardiometabolic risk. Indeed, population-based studies have described associations between retinopathy and future cardiovascular events, including in non-diabetic populations (Cheung, Rogers, et al., 2007; Cheung, Wang, et al., 2007; Klein et al., 2000; Liew, Wong, Mitchell, Cheung, & Wang, 2009; Wong, Klein, Couper, et al., 2001; Wong, Klein, Nieto, et al., 2003; Wong et al., 2002; Wong, Rosamond, et al., 2005). Only a small number of prospective studies have evaluated whether retinopathy in non-diabetic individuals predicts subsequent risk of diabetes (Klein, Klein, Moss, & Wong, 2006; Wong, Mohamed, Klein, & Couper, 2006). In this context the presence of retinopathy may serve as a readily accessible marker of risk of progression and allow more aggressive treatments to be targeted to those at higher risk of metabolic progression. The data on this question are sparse.
The Early Diabetes Intervention Program (EDIP) was a 5-year prospective randomized controlled trial evaluating the effect of acarbose versus placebo on a background of diet/exercise recommendations to delay progression of fasting glucose in a population with screen-detected, early type 2 diabetes. In the current study, we examined the relationships of retinopathy at baseline to the 5-year progression of fasting plasma glucose. Parallel analyses were performed with measures of nephropathy and neuropathy.
Research Design and Methods
EDIP was carried out between 1998 and 2004 at Indiana University School of Medicine and Washington University School of Medicine. The eligibility criteria, study design, and methods have been reported elsewhere (Kirkman et al., 2006). Participants were recruited from a population at high risk of diabetes, consisting of men and women at least 25 years of age with obesity, a history of gestational diabetes, or a family history of diabetes. They were eligible for the study if they had fasting plasma glucose (FPG) measurement between 105 mg/dl (5.5 mmol/l) and 140 mg/dl (7.8 mmol/l), the diagnostic FPG cutoff for diabetes at time of study inception, plus a 2-h post load plasma glucose (75-g oral glucose tolerance test) measurement ≥200 mg/dl (11.1 mmol/l). Major exclusion criteria included recent treatment for cancer, recent major atherosclerotic events, and chronic infectious diseases. Full details of study inclusion/exclusion criteria have been previously published (Kirkman et al., 2006).
Eligible participants received either acarbose or an identical placebo based on a blinded randomization stratified by site. Acarbose was titrated up to the maximum dose of 100 mg three times a day with meals, or the maximum tolerated dose, with matched dose increments among placebo treated individuals. At initiation of randomized study treatment, all subjects received diabetic diet counseling by a registered dietician, and were provided with standard of care recommendations for exercise (Kirkman et al., 2006). This advice was reinforced at quarterly visits.
At baseline, each participant completed a standardized medical history, physical examination, electrocardiogram, seven-field fundus photography of both eyes, oral glucose tolerance test, laboratory testing, and 24-h urine collection for measurement of creatinine and albumin concentrations. Participants were seen quarterly for measurement of fasting plasma glucose concentration. The primary outcome for EDIP was the time to transition of fasting plasma glucose >140 mg/dL (7.8 mmol/L), confirmed on a repeat measurement at the next quarterly visit. This event was termed ‘progression of fasting glucose’, and provided a metric of worsening glycemia amenable to time-to-event analyses. Participants who met criteria for progression did not undergo any further study measurements.
The study protocol was approved by the institutional review boards of both institutions, and each patient signed an informed consent form for screening and for the trial.
Assessment of microvascular disease
Retinal photographs were acquired using a standardized protocol. Seven standard photographic fields were taken in each eye, by experienced retinal photographers who underwent central training and certification by the reading center in Madison WI. A 30° Zeiss FF series fund camera with Kodachrome 25 Daylight film was used, as specified by the reading center. Printed slide mages were read at a central unit using standardized methodology, with readers blinded to all participant information. Retinopathy scores and presence or absence of retinopathy was defined according to a simplified version of the Wisconsin grading system (ETDRS score ≥20 in either eye defining the categorical presence of retinopathy).
Nephropathy was evaluated using the 24 hour urinary albumin:creatinine ratio using a Cobas MIRA autoanalyzer (Roche, Nutley NJ). A ratio ≥30 mg/g/24hr was used to define the categorical presence of nephropathy (Kramer & Molitch, 2005). All laboratory measures were performed at a central study lab at Indiana University.
Neuropathy was evaluated using 10-g monofilament testing on both feet. This testing was performed by physicians, and graded by the number of touches successfully identified by participants out of a total 10 per foot, with the presence of neuropathy determined if fewer than 8 touches were detected on either foot (Feng, Schlosser, & Sumpio, 2009).
Anthropomorphic and Laboratory measurements
Weight and height were measured with participants wearing light clothing, and the body mass index (BMI) was calculated as weight divided by the square of the height (kg/m2). Blood pressure was measured twice on the right arm while sitting, using a calibrated mercury sphygmomanometer. The average of these measurements was used for analysis.
Glucose concentrations were determined using a glucose oxidase method (YSI, Yellow Springs, OH). Insulin was measured using a radioimmune assay (Linco Research, St Charles, MO). HbA1c was measured using an immunoturbidometric assay (Roche Diagnostics, Indianapolis, IN). Total and HDL cholesterol and triglycerides were measured using an enzymatic end-point assay (Roche Diagnostics). LDL cholesterol concentration was derived using the Friedewald calculation if the value for triglycerides was <400 mg/dl. Non-esterified fatty acids were measured using a colorimetric method (Wako Diagnostics, Richmond VA). Beta cell function was quantified as the Insulinogenic Index, (IGI:[ins(30)-ins(0)]/[gluc(30)-gluc(0)]). Insulin resistance was estimated using HOMA-IR and 1/(fasting insulin). Disposition Index was calculated as IGI multiplied by 1/(fasting insulin). Glucose and insulin area under the curve following the oral glucose challenge were calculated as the excursion above baseline, using the trapezoidal rule.
Statistical Analysis
Cox proportional hazards models were used to assess the effect of baseline retinopathy on progression of the fasting plasma glucose. We adjusted for relevant metabolic, demographic and anthropometric baseline variables and change in variables in multivariable model. Change was calculated as [Yr1 – Yr0], because this provided both the maximal on-study effect and largest available dataset. A threshold significance value for these univariate relationships of p ≤0.1 was applied to select variables for inclusion in the multivariable models. Multivariable models were first constructed using only baseline variables, and then incorporating baseline and change variables that met these criteria.
All statistical analyses were performed with SAS statistical software 9.4 SAS institute Inc. Cary, NC. Statistical significance was pre-specified at p<0.05.
Results
The initial enrolled cohort of the EDIP trial comprised 216 participants. 196 subjects underwent at least two consecutive quarterly visits after the baseline visit, sufficient for determination of the pre-specified study endpoint. Of these, 2 subjects did not have information on baseline retinopathy. Hence, our final sample consisted of 194 subjects to evaluate the prospective relationship of baseline retinopathy with progression of fasting plasma glucose. Due to censoring at the time of progression (6) and individuals lost to follow-up (42) in the first year, analyses incorporating change in baseline variables to year 1 of follow-up included 164 subjects. After the first year the study retention was much improved, with the majority of censoring coming from progression events. Median duration on study was 30 months.
At the baseline evaluation 24/194 subjects with retinal photographs had retinopathy (12.4%). In this population, the mean (SD) ETDRS scores in right and left eye respectively were 18.9 (16.6) and 17.3 (7.2) respectively among those with retinopathy, and 10.0 (0.0) and 10.0 (0.07) respectively among those without retinopathy. The scores for the worst eye among those meeting criteria for retinopathy were 23.7 (14.6), and the distribution of pairs of scores for both eyes were 10/10 n=168; 20/10 n=22; 20/20 n=2; and >20/20 n=2. These 194 subjects did not differ in key anthropomorphic or metabolic variables from the complete set of subjects included at initiation of the study (not shown). The prevalence of neuropathy among individuals who underwent baseline assessments, regardless of subsequently undergoing randomization, was 19/225 evaluable participants (8.4%), and of nephropathy was 10/221 evaluable participants (4.5%). In this population there was little overlap of individuals affected by these conditions: Of 199 individuals with evaluable data for all 3 domains, 51 had evidence of one microvascular disease, 3 had two such conditions and none had all three.
Table 1 presents demographic, anthropomorphic and metabolic variables at baseline, according to the presence or absence of retinopathy at baseline. None of these variables differed by presence or absence of retinopathy at baseline. Subjects with neuropathy at baseline had higher weight (113.8 (23.2) vs 96.9 (15.6) kg, p= 0.005) compared to subjects without neuropathy. Subjects with nephropathy at baseline had higher systolic blood pressure (149.0 (17.3) vs 132.5 (17.4), p=0.01) compared to subjects without nephropathy. Notable in this set of analyses were the very low mean HbA1c (6.2%), and a lack of differences in age, fasting plasma glucose, or blood pressure by retinopathy status (Table 1) or neuropathy status (not shown).
Table 1.
Baseline characteristics of the EDIP population by retinopathy, neuropathy, and nephropathy status
| Baseline characteristics | Retinopathy (n=194) | ||
|---|---|---|---|
| Yes (n=24) | No (n=170) | p-value | |
| N (%) | N (%) | ||
| Treatment | 0.41 | ||
| • Acarbose | 10 (41.7) | 86 (50.6) | |
| • Placebo | 14 (58.3) | 84 (49.4) | |
| Sex | 0.59 | ||
| • Male | 7 (29.2) | 59 (34.7) | |
| • Female | 17 (70.8) | 111 (65.3) | |
| Mean±SD | Mean±SD | ||
| Age (years) | 54.6±11.6 | 54.1±11.5 | 0.85 |
| Weight (kg) | 97.9±18.3 | 97.8±20.4 | 0.98 |
| BMI (kg/m2) | 35.3±7.5 | 34.8±6.6 | 0.74 |
| FPG (mmol/min) | 6.8±0.8 | 6.7±0.8 | 0.67 |
| OGTT 30 min glucose (mmol/L) | 10.8±2.2 | 10.9±1.9 | 0.89 |
| OGTT 60 min glucose (mmol/L) | 13.3±1.7 | 13.4±1.9 | 0.74 |
| OGTT 120 min glucose (mmol/L) | 12.7±1.62 | 13.1±1.7 | 0.27 |
| HbA1C (%) | 6.3±0.7 | 6.0±1.8 | 0.22 |
| Systolic BP (mmHg) | 130.5±14.0 | 133.4±17.9 | 0.46 |
| Diastolic BP (mmHg) | 75.7±12.2 | 75.3±11.0 | 0.87 |
| Lipids (mmol/L) | |||
| •Total Cholesterol | 5.0±1.1 | 5.0±1.0 | 0.96 |
| •HDL Cholesterol | 1.0±0.3 | 1.0±0.2 | 0.40 |
| •LDL Cholesterol | 3.1±1.0 | 3.0±0.9 | 0.80 |
| •Triglycerides | 1.9±0.9 | 2.2±1.2 | 0.22 |
| HOMA-IR (U) | 5.4±4.0 | 5.6±4.0 | 0.75 |
| Fasting Insulin (pmol/L) | 127.9±84.5 | 138.3±85.9 | 0.59 |
| Insulinogenic Index (pmol/mmol) | 53.8±72.4 | 68.7±78.6 | 0.39 |
| Disposition Index (U) | 0.4±0.4 | 0.6±0.4 | 0.25 |
| Fasting NEFA (mmol/L) | 627.6±151.5 | 568.9±204.3 | 0.18 |
Note: P-values reported are from chi-square analysis for categorical variables, or unpaired t-test for continuous variables. Sample sizes in the column headings represent the number of evaluable participants for each microvascular complication.
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; HTN, hypertension; BP, blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; HbA1C, hemogloblin A1C; HOMA-IR, homeostasis model of insulin resistance; NEFA, non-esterified fatty acids; OGTT, oral glucose tolerance test.
In univariate Cox analysis, the presence of retinopathy at baseline was associated with a doubling of risk of progression of fasting plasma glucose (HR 2.02; 95%CI 1.05–3.89, Table 2). We next performed multivariable Cox analyses, first adjusting the retinopathy effect for relevant variables one by one (Table 2 Stage 1 Models). In these models, baseline retinopathy remained significantly associated with progression of fasting glucose while individually adjusting for age (p=0.039), weight (p=0.026), fasting plasma glucose (p=0.030), post challenge glucose values (p=0.060, p=0.050, and p=0.034 for values at 30 min, 60 min, and 120 min respectively), systolic and diastolic blood pressure (p=0.038 and p=0.037 respectively), or HbA1C (p=0.031). Similar analyses were performed incorporating individual Year 1 – Year 0 change variables individually with retinopathy status (Table 2, Stage 1 Models). Each of these change terms individually caused the retinopathy term to lose statistical significance, with adjusted hazard ratio point estimates ranging from 1.54 to 1.92.
Table 2.
Cox proportional hazards model evaluating effect of baseline retinopathy on progression of fasting glucose
| FPG Progression | ||
|---|---|---|
| p-value | Retinopathy HR (95% CI) | |
| N = 194 | ||
| Retinopathy (0=No, 1=Yes) | 0.035 | 2.02 (1.05 – 3.89) |
| N = 194 | ||
| Stage 1: Baseline variables | ||
| Retinopathy + treatment group (acarbose vs. placebo) | 0.046 | 1.96 (1.01 – 3.79) |
| Retinopathy + Age (years) | 0.039 | 1.99 (1.04 – 3.84) |
| Retinopathy + Weight (kg) | 0.026 | 2.12 (1.10 – 4.08) |
| Retinopathy + FPG (mmol/L) | 0.030 | 2.08 (1.08 – 4.01) |
| Retinopathy + OGTT 30 min glucose (mmol/L) | 0.060 | 1.88 (0.97 – 3.63) |
| Retinopathy + OGTT 60 min glucose (mmol/L) | 0.050 | 1.93 (1.01 – 3.72) |
| Retinopathy + OGTT 120 min glucose (mmol/L) | 0.034 | 2.04 (1.06–3.92) |
| Retinopathy + Fasting NEFA (mmol/L) | 0.11 | 1.73 (0.89 – 3.38) |
| Retinopathy + HbA1c (%) | 0.031 | 2.06 (1.07 – 3.98) |
| Retinopathy + SBP (mmHg) | 0.038 | 2.01 (1.04–3.88) |
| Retinopathy + DBP (mmHg) | 0.037 | 2.02 (1.05–3.90) |
| N = 164 | ||
| Stage 1: Change variables | ||
| Retinopathy + Δ FPG (mmol/L) | 0.16 | 1.67 (0.82–3.42) |
| Retinopathy + Δ OGTT Glucose (30 min – 0 min) (mmol/L) | 0.08 | 1.92 (0.93 – 3.94) |
| Retinopathy + Δ OGTT Glucose (60 min – 0 min) (mmol/L) | 0.11 | 1.80 (0.88 – 3.69) |
| Retinopathy + Δ OGTT AUC Excursion (mmol/L * min) | 0.12 | 1.75 (0.86 – 3.59) |
| Retinopathy + Δ Fasting Insulin (pmol/L) | 0.27 | 1.55 (0.71 – 3.40) |
| Retinopathy + Δ HbA1C (%) | 0.26 | 1.54 (0.73 – 3.22) |
| Retinopathy + Δ weight | 0.13 | 1.74 (0.85–3.55) |
| N = 194 | ||
| Stage 2 Model 1: Multivariable analysis with baseline variables | HR (95%CI) per variable | |
| Retinopathy | 0.12 | 1.80 (0.87 – 3.76) |
| Treatment group (acarbose vs. placebo) | 0.60 | 1.16 (0.67–2.03) |
| Age | 0.03 | 0.97 (0.94 – 1.00) |
| Weight | 0.12 | 1.01 (1.00 – 1.02) |
| FPG | <0.0001 | 3.07 (1.81 – 5.20) |
| OGTT 30 min glucose | 0.27 | 1.10 (0.93 – 1.31) |
| OGTT 60 min glucose | 0.36 | 0.91 (0.75–1.11) |
| OGTT 120 min glucose | 0.32 | 1.09 (0.92–1.30) |
| HbA1c | 0.42 | 1.28 (0.85 – 1.93) |
| SBP | 0.24 | 0.99 (0.97–1.01) |
| DBP | 0.76 | 1.04 (0.98–1.03) |
| N = 164 | ||
| Stage 2 Model 2: Multivariable analysis with baseline and change variables | ||
| Retinopathy | 0.78 | 1.14 (0.45 – 2.90) |
| Treatment group | 0.96 | 0.98 (0.53–1.82) |
| Age | 0.14 | 0.98 (0.95 – 1.01) |
| Weight | 0.19 | 1.01 (0.99 – 1.03) |
| FPG | 0.10 | 1.67 (0.90 – 3.11) |
| OGTT 30 min glucose | 0.0002 | 1.55 (1.24–1.95) |
| OGTT 60 min glucose | 0.009 | 0.76 (0.61–0.93) |
| OGTT 120 min glucose | 0.24 | 1.01 (1.00–1.02) |
| HbA1c | 0.77 | 1.09 (0.61 – 1.94) |
| SBP | 0.057 | 0.98 (0.97–1.00) |
| DBP | 0.19 | 1.02 (0.99–1.05) |
| Δ OGTT Glucose (30 min – 0 min) (mmol/L) | <0.0001 | 1.58 (1.34–1.87) |
Abbreviations: Δ, delta; AUC, area under the curve; FPG, fasting plasma glucose; HbA1C, hemogloblin A1C; NEFA, non-esterified fatty acids; OGTT, oral glucose tolerance test; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, hazard ratio; CI, confidence interval
The next analyses used multivariable cox proportional hazards modeling incorporating all baseline variables that met criteria for inclusion (Stage 2 Model 1), and incorporating all qualifying baseline and change variables (Stage 2 Model 2). In the analysis incorporating baseline variables, retinopathy status was no longer statistically significantly associated with progression of glucose, with the point estimate of the hazard ratio for this term reduced to 1.80. In this model, younger age (p=0.03) and higher fasting plasma glucose (p<0.0001) were the dominant predictors of progression of fasting glucose.
The analysis incorporating baseline and change variables also removed the statistical significance for retinopathy status, in this instance with a more profound effect on the associated point estimate of the hazard ratio (Table 2 Stage 2 Model 2). In this analysis, higher 30 min post glucose challenge (p=0.0002), lower 60 min post glucose challenge (p=0.009), and increase in 30 min post glucose challenge change (p<0.0001) were the persisting significant determinants for progression of fasting glucose. In the multivariable analyses mutually adjusting for baseline variables, the estimated hazard ratio associated with baseline retinopathy was not strongly reduced, although this term did not retain significance. In contrast the multivariable modeling incorporating baseline and Year 1 change variables produced a materially reduced estimated hazard ratio for the retinopathy term.
Baseline neuropathy and nephropathy were not associated with progression of fasting glucose. The hazard ratio associated with the presence of neuropathy was 1.39 (95% CI 0.50–03.84), p=0.53. The hazard ratio associated with nephropathy was 0.98 (95% CI 0.31 – 3.12), p=0.97.
Discussion
The EDIP population was selected by virtue of having screen-detected Type 2 diabetes, specifically exhibiting post-challenge hyperglycemia but with less overt dysregulation of fasting glucose. Mean baseline HbA1c in this group at study entry was 6.2%, reflecting the very early stage of diabetes in this screen-detected population. Despite this early diabetes condition, the population had a 12.4% prevalence of retinopathy, an 8.4% prevalence of neuropathy and 4.5% prevalence of nephropathy at baseline (Kirkman et al., 2006). Here we have shown that having retinopathy at baseline was significantly associated with an increased risk of progression of fasting glucose (p=0.035, hazard ratio [95% confidence interval] of 2.02 [1.05 – 3.89]). In contrast there was no association of nephropathy or neuropathy at baseline with risk of progression of fasting glucose. The association of retinopathy with progression of fasting glucose was robust to individual adjustments for age, baseline weight, fasting glucose and HbA1c, but lost its significance in multivariable models incorporating these baseline and change in factors at the end of first year. This suggests that an assessment of retinopathy could be of value in initial assessments of risk of metabolic worsening in early diabetes, adding information beyond the risk associated with age and glycemia.
An association of retinal disease with prospective risk of metabolic worsening is not widely known, although a small number of publications have evaluated these relationships. Retinal arteriolar narrowing has been proposed as a predictor for development of diabetes (Wong, Shankar, Klein, Klein, & Hubbard, 2005). Similarly, the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study showed that narrower retinal arterioles were associated with increased risk of developing diabetes, independent of FPG and SBP (Nguyen et al., 2008). More traditional diabetes-related retinopathy (i.e. dot-blot hemorrhage, macular edema) has also been associated with risk of metabolic progression. In the ARIC (Atherosclerosis Risk In Communities) study, the presence of ETDRS-defined retinopathy was associated with subsequent risk for diabetes in individuals with a family history of diabetes (Wong et al., 2006). In the Blue Mountains Eye study, 3.5% developed diabetes over 5 years among 195 individuals having retinopathy lesions at baseline (Cugati et al., 2006). In the Beaver Dam Eye Study, retinopathy was present in 7.3% of non-diabetic persons and these people were more likely to subsequently develop diabetes over the next 15 years (OR 1.70, 95%CI 1.17–2.48, p=0.005) (Klein et al., 2006). In a cross-sectional study by Chowdhury et al. retinopathy in newly diagnosed Type 2 diabetes (mean HbA1c) was associated with lower beta cell responsiveness, insulin resistance, and insulin levels, and elevated fasting and postprandial glucose (Roy Chowdhury et al., 2016). In our study we see an association of retinopathy with progression of fasting plasma glucose, but we found no association found between retinopathy and baseline levels of beta cell function, insulin resistance, and insulin levels. This difference in observed associations is presumably due to the different underlying patient populations, with differing ranges of the variables being compared.
Our observations extend and augment these prior reports. We confirm the prospective relationship of baseline retinopathy lesions with increased risk of progressive dysglycemia, within a group with early screen-detected diabetes. Further, we provide quantification of the strength of the relationship of retinopathy versus other factors that are predictive of glycemic progression. Our study used more rigorous ascertainment of glycemic progression than has been used in prior reports, and applied a research-standard evaluation of retinopathy using readers blinded to participant characteristics. By virtue of the strength of our outcome assessments, many of the components of our multivariable analyses were more robustly ascertained than has been possible in the above population studies reporting this association.
The mechanism(s) underlying the association of prevalent retinopathy with progressive metabolic worsening are unknown. It is possible that the presence of retinopathy may simply be informing us that the affected individual has experienced a greater magnitude or duration of exposure to elevated glucose levels than is evident in their current glucose values. Since glucose itself is a strongly dominant predictor of progressive dysglycemia in this dataset (Table 2) and others (Cameron et al., 2008; Sattar et al., 2008; Wilson, D’Agostino, Parise, Sullivan, & Meigs, 2005; Wilson et al., 2007), the prior existence of important levels of dysglycemia would be a logical connection with retinopathy. A recent report found an inverse association of fasting measures of β-cell function with retinopathy prevalence (Kuo et al., 2014), consistent with this notion. However, in our population there was no difference in the insulinogenic index or the disposition index at baseline by retinopathy status (Table 1), suggesting that impaired β-cell function may be an insufficient explanation. It is also possible that the presence of retinopathy identifies an individual with an increased microvascular susceptibility to glycemia, such that even modest elevations promote disease progression. A third possibility is that the presence of retinopathy is due to some shared factor arising with the dysmetabolic state that is common to vascular and metabolic worsening (i.e. a ‘common soil’). Our analyses do not distinguish among these three possibilities but it is worth noting that the observations in adjusted univariate analyses suggest that the effect of retinopathy on risk of progression may be independent of glucose. Further evidence for a glucose-independent relationship is the lack of parallel observations in our cohort in the association of nephropathy and neuropathy with progression of glucose, although factors such as detection sensitivity and glucose exposure thresholds for disease may be masking true underlying associations. Future studies will need to be designed to specifically to address these questions.
Limitations
The current evaluations are post-hoc analyses of data collected in the context of a clinical trial, and therefore must be viewed as hypothesis-generating. The randomized treatment did not affect the relationship between retinopathy and progression of fasting glucose, but nevertheless this dataset differs from more traditional epidemiologic evaluations of these prospective relationships. The methodologies used for detection and quantification of retinopathy and nephropathy were arguably more sensitive than the standard methods used to detect and quantify neuropathy. Nevertheless, the methods used for retinopathy and nephropathy were of high quality and all methods are parallel to those used in clinical practice, helping ensure that these observations can be interpreted in the context of routine clinical observations. The reduced number of subjects with evaluable data for analyses incorporating Year 1 data is in part an artifact of the study design, where subjects who met the study endpoint were no longer followed. Despite this a large majority of the original enrollees had data evaluable for these analyses, and this subgroup was clearly representative of the baseline cohort. On this basis we conclude that these analyses are representative of individuals with similar degrees of dysglycemia. The notion of assessing retinopathy as a biomarker or risk factor for diabetes has some appeal in that it is relatively simple, non-invasive, and well tolerated. Nevertheless, such an effort would require including retinopathy assessments, with associated resource needs and costs, in the overall risk factor profile of individuals being assessed for risk of glycemic worsening. This may not be feasible or realistic in the general population with current methods, but could be of value in clinical trials of diabetes prevention or β-cell function preservation.
Conclusions
In this population with early, screen-detected type 2 diabetes, we found that the presence of retinopathy lesions at baseline was associated with a two-fold increased risk of progression of fasting plasma glucose. Other microvascular disease measures that including reduced monofilament sensation and elevated urinary albumin:creatinine ratio at baseline were not predictors of progression of fasting plasma glucose, although the prevalence of these conditions was low in this population which presumably limited our power for demonstrating such relationships even if they truly existed. Early detection of retinopathy may help individualize more aggressive therapy to prevent progressive metabolic worsening in early diabetes.
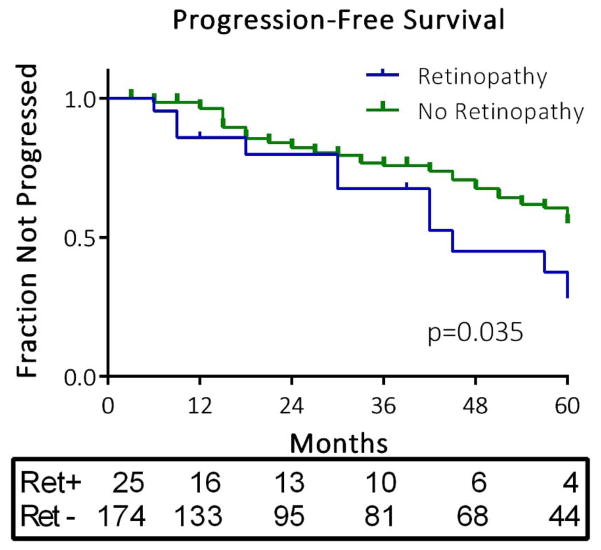
Figure 1.
Time course of progression of fasting glucose by retinopathy status at baseline. The survival curve depicts time to censoring events and event-free survival among participants with and without retinopathy at baseline. The p value represents the effect of baseline retinopathy on event-free survival in a Cox proportional hazards model. The numbers of individuals remaining in each group at each annual time point across the 5 years of observation are presented in the box below the figure.
Acknowledgments
This work was supported by an investigator-initiated grant from Bayer with additional support from the National Institutes of Health (grants P60 DK20542, P60 DK20579 and GCRC M01RR00750). Data analysis and manuscript preparation and presentation were performed completely independently from the study sponsor. The study was registered on a public accessible database (www.clinicaltrials.gov; NCT01470937).
We gratefully acknowledge the contributions of our study participants, and those of the study staff and CRC staff at the participating institutions.
Footnotes
Disclosures/conflict of interests: None by any authors
Author contributions: YP and KM wrote the manuscript. YP performed statistical analyses. RC and MK provided study data. TH, RC and MK edited the manuscript and contributed to the discussion.
References
- Cameron AJ, Magliano DJ, Zimmet PZ, Welborn TA, Colagiuri S, Tonkin AM, Shaw JE. The metabolic syndrome as a tool for predicting future diabetes: the AusDiab study. J Intern Med. 2008;264(2):177–186. doi: 10.1111/j.1365-2796.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- Cheung N, Rogers S, Couper DJ, Klein R, Sharrett AR, Wong TY. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke. 2007;38(2):398–401. doi: 10.1161/01.STR.0000254547.91276.50. [DOI] [PubMed] [Google Scholar]
- Cheung N, Wang JJ, Klein R, Couper DJ, Sharrett AR, Wong TY. Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2007;30(7):1742–1746. doi: 10.2337/dc07-0264. [DOI] [PubMed] [Google Scholar]
- Cugati S, Cikamatana L, Wang JJ, Kifley A, Liew G, Mitchell P. Five-year incidence and progression of vascular retinopathy in persons without diabetes: the Blue Mountains Eye Study. Eye (Lond) 2006;20(11):1239–1245. doi: 10.1038/sj.eye.6702085. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24(2):137–144. doi: 10.1111/j.1464-5491.2007.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg. 2009;50(3):675–682. 682 e671. doi: 10.1016/j.jvs.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Kirkman MS, Shankar RR, Shankar S, Shen C, Brizendine E, Baron A, McGill J. Treating postprandial hyperglycemia does not appear to delay progression of early type 2 diabetes: the Early Diabetes Intervention Program. Diabetes Care. 2006;29(9):2095–2101. doi: 10.2337/dc06-0061. [DOI] [PubMed] [Google Scholar]
- Klein R. Retinopathy in a population-based study. Trans Am Ophthalmol Soc. 1992;90:561–594. [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Wang Q. Hypertension and retinopathy, arteriolar narrowing, and arteriovenous nicking in a population. Arch Ophthalmol. 1994;112(1):92–98. doi: 10.1001/archopht.1994.01090130102026. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Wong TY. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2006;104:98–107. [PMC free article] [PubMed] [Google Scholar]
- Klein R, Sharrett AR, Klein BE, Chambless LE, Cooper LS, Hubbard LD, Evans G. Are retinal arteriolar abnormalities related to atherosclerosis?: The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20(6):1644–1650. doi: 10.1161/01.atv.20.6.1644. [DOI] [PubMed] [Google Scholar]
- Kramer H, Molitch ME. Screening for kidney disease in adults with diabetes. Diabetes Care. 2005;28(7):1813–1816. doi: 10.2337/diacare.28.7.1813. [DOI] [PubMed] [Google Scholar]
- Kuo JZ, Guo X, Klein R, Klein BE, Weinreb RN, Genter P, … Ipp E. Association of fasting insulin and C peptide with diabetic retinopathy in Latinos with type 2 diabetes. BMJ Open Diabetes Res Care. 2014;2(1; e000027):1–8. doi: 10.1136/bmjdrc-2014-000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA, … Dawber TR. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24(Suppl):335–610. [PubMed] [Google Scholar]
- Liew G, Wong TY, Mitchell P, Cheung N, Wang JJ. Retinopathy predicts coronary heart disease mortality. Heart. 2009;95(5):391–394. doi: 10.1136/hrt.2008.146670. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Wang JJ, Islam FM, Mitchell P, Tapp RJ, Zimmet PZ, … Wong TY. Retinal arteriolar narrowing predicts incidence of diabetes: the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes. 2008;57(3):536–539. doi: 10.2337/db07-1376. [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury S, Thomas RL, Dunseath GJ, Peter R, Rees DA, North RV, … Owens DR. Diabetic Retinopathy in Newly Diagnosed Subjects With Type 2 Diabetes Mellitus: Contribution of beta-Cell Function. J Clin Endocrinol Metab. 2016;101(2):572–580. doi: 10.1210/jc.2015-2203. [DOI] [PubMed] [Google Scholar]
- Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, … Wannamethee SG. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371(9628):1927–1935. doi: 10.1016/S0140-6736(08)60602-9. [DOI] [PubMed] [Google Scholar]
- Sharp PS, Chaturvedi N, Wormald R, McKeigue PM, Marmot MG, Young SM. Hypertensive retinopathy in Afro-Caribbeans and Europeans. Prevalence and risk factor relationships. Hypertension. 1995;25(6):1322–1325. doi: 10.1161/01.hyp.25.6.1322. [DOI] [PubMed] [Google Scholar]
- Svardsudd K, Wedel H, Aurell E, Tibblin G. Hypertensive eye ground changes. Prevalence, relation to blood pressure and prognostic importance. The study of men born in 1913. Acta Med Scand. 1978;204(3):159–167. [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167(10):1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, … Sharrett AR. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46(1):59–80. doi: 10.1016/s0039-6257(01)00234-x. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, … Tielsch JM. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110(5):933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, … Mosley TH. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288(1):67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, Manolio TA, Hubbard LD, Marino EK, … Siscovick DS. The prevalence and risk factors of retinal microvascular abnormalities in older persons: The Cardiovascular Health Study. Ophthalmology. 2003;110(4):658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- Wong TY, Mohamed Q, Klein R, Couper DJ. Do retinopathy signs in non-diabetic individuals predict the subsequent risk of diabetes? Br J Ophthalmol. 2006;90(3):301–303. doi: 10.1136/bjo.2005.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Rosamond W, Chang PP, Couper DJ, Sharrett AR, Hubbard LD, … Klein R. Retinopathy and risk of congestive heart failure. JAMA. 2005;293(1):63–69. doi: 10.1001/jama.293.1.63. [DOI] [PubMed] [Google Scholar]
- Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Retinal arteriolar narrowing, hypertension, and subsequent risk of diabetes mellitus. Arch Intern Med. 2005;165(9):1060–1065. doi: 10.1001/archinte.165.9.1060. [DOI] [PubMed] [Google Scholar]
- Yu T, Mitchell P, Berry G, Li W, Wang JJ. Retinopathy in older persons without diabetes and its relationship to hypertension. Arch Ophthalmol. 1998;116(1):83–89. doi: 10.1001/archopht.116.1.83. [DOI] [PubMed] [Google Scholar]