Figure 6.
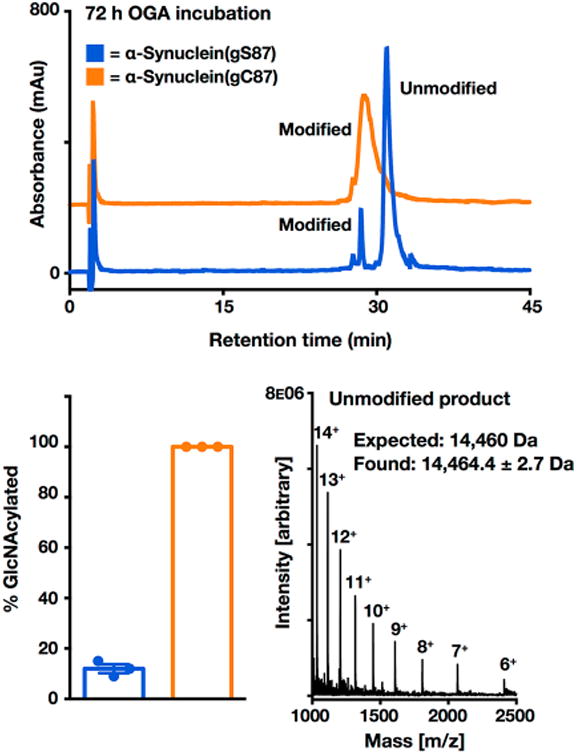
S-GlcNAcylation of α-synuclein is enzymatically stable. α-Synuclein(gS87) or α-synuclein(gC87) (25 μM, in PBS at pH 7.4) was incubated in triplicate with OGA (1 μM) at 37 °C for 72 h. S-GlcNAc is offset in the y-direction for the sake of clarity. mAU indicates milliabsorbance units. Hydrolysis of GlcNAcylated α-synuclein was analyzed by HPLC at the 72 h time point. Deglycosylation was quantitated by area percent using high-performance liquid chromatography (HPLC) at 214 nm, and the deglycosylated product was characterized by ESI-MS. Results are the mean ± the standard error of the mean of three separate biological experiments. RP-HPLC conditions were 35–60% buffer B over 60 min; buffer A consisted of 0.1% TFA in H2O, and buffer B consisted of 0.1% TFA and 90% ACN in H2O.
