Figure 7.
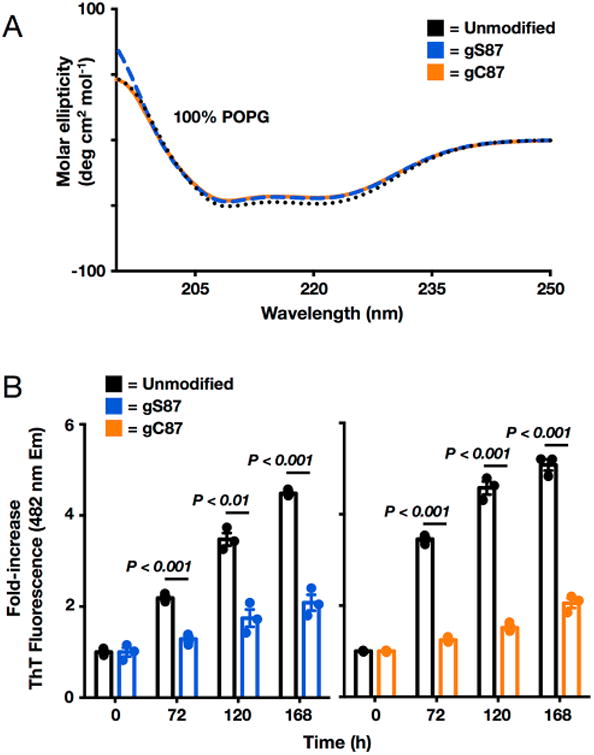
S-GlcNAcylation has effects identical to those of O-GlcNAcylation on the membrane binding and aggregation of α-synuclein. (A) Neither O-GlcNAcylation nor S-GlcNAcylation at residue 87 affects α-synuclein membrane binding. Recombinant α-synuclein, α-synuclein(gS87), or α-synuclein(gC87) was incubated with a 100-fold excess of POPG preformed vesicles and analyzed using circular dichroism (CD). All of the proteins gave essentially indistinguishable CD spectra consistent with the formation of an extended α-helix. POPG denotes 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)]. (B) O-GlcNAcylation and S-GlcNAcylation are equally inhibitory toward a-synuclein aggregation. Recombinant α-synuclein, α-synuclein(gS87), or α-synuclein(gC87) was subjected to aggregation conditions (25 μM concentration and agitation at 37 °C) for the indicated lengths of time before analysis of aliquots by ThT fluorescence (λex = 450 nm, and λem = 482 nm). The y-axis shows the fold increase in fluorescence compared with the corresponding protein at time zero. Error bars represent the standard error of the mean from the mean of three biological replicates, and statistical significance was calculated using a two-tailed Student's t test.
