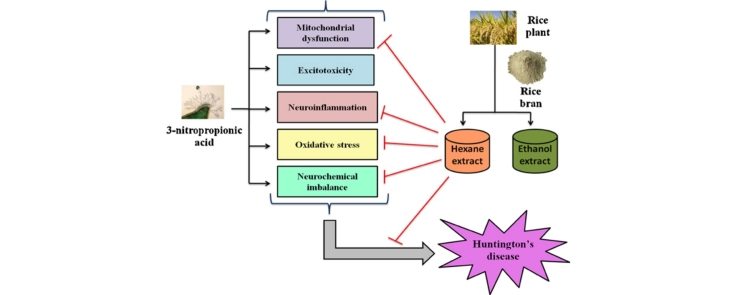
Graphical abstract
Keywords: 3-Nitropropionic acid, Huntington's chorea, Oxidative stress, Rice bran, Mitochondrial dysfunction, Basal ganglia
Abstract
Huntington's disease (HD) is a neurodegenerative disorder, characterized by progressive motor and non-motor dysfunction due to degeneration of medium spiny neurons in striatum. 3-Nitropropionic acid is commonly used to induce the animal model of HD. Rice bran is supposed to have beneficial effects on mitochondrial function. The present study has been designed to explore the effect of rice bran extract against 3-Nitropropionic acid induced neurotoxicity in rats. 3-Nitropropionic acid (10 mg/kg, i.p) was administered systemically for 21 days. Hexane and ethanol extract of rice bran were prepared using Soxhlation. Hexane (250 mg/kg) and ethanol extract (250 mg/kg) were administered per os for 21 days in 3-NP treated groups. Behavioral parameters (body weight, grip strength, motor coordination, locomotion) were conducted on 7th, 14th and 21st day. Animals were sacrificed on 22nd day for biochemical, mitochondrial dysfunction (Complex II), neuroinflammatory and neurochemical estimation in striatum. This study demonstrates significant alteration in behavioral parameters, oxidative burden (increased lipid peroxidation, nitrite concentration and decreased glutathione), mitochondrial function (decreased Complex II enzyme activity), pro-inflammatory mediators and neurochemical levels in 3-nitropropionic acid treated animals. Administration of hexane and ethanol extract prevented the behavioral, biochemical, neuroinflammatory (increased TNF-α, IL-1β and IL-6) and neurochemical alterations (decreased dopamine, norepinephrine, serotonin, 5-hydroxy indole acetic acid, GABA and increased 3,4-dihydro phenyl acetaldehyde, homovanillic acid and glutamate levels) induced by 3-nitropropionic acid. The outcomes of present study suggest that rice bran extract is beneficial and might emerge as an adjuvant or prophylactic therapy for treatment of HD like symptoms.
1. Introduction
Huntington's disease (HD) is a progressive and fatal neurodegenerative disorder characterized by striatal specific degeneration of GABA-ergic medium spiny neurons (MSNs) and associated with motor, psychiatric and cognitive disturbances [1], [2]. The exact pathogenic mechanism underlying HD has not been elucidated yet, but mitochondrial dysfunction, excitotoxicity, neuroinflammation, oxidative stress, neurochemical imbalance and apoptosis are most well accepted mechanisms [3], [4]. Tetrabenazine is the only drug that has been approved by US FDA for treatment of chorea related to HD [5]. Various toxins and genetic animal models are being used for testing of novel and safe therapies.
3-NP is a mycotoxin, obtained from fungi and plants and is widely used animal model for HD. It acts by irreversibly inhibiting mitochondrial complex II (Succinate dehydrogenase) enzyme of electron transport chain [6], [7]. Intake of 3-NP in humans and rodents produces motor abnormalities including dystonia, torsion spasms, involuntary jerky movements, facial grimaces and cognitive impairment [3]. 3-NP toxicity leads to diminution of adenosine triphosphate (ATP) and energy depletion, altered calcium homeostasis, excitotoxicity, free radical generation and neuronal death [8].
Rice bran is the by-product of rice milling process and contains about 10% of rough rice grain and 18⿿22% oil [9]. It mainly contains γ-oryzanol, coumaric acid, ferulic acid, caffeic acid, phytic acid, carotenoids, tocopherols, tocotrienols and phytosterols. Rice bran has been found to beneficial against inflammation, hyperlipidemia, coronary heart disease, oxidative stress, cancer, type II diabetes, Alzheimer's disease and aging. Therefore, the present study was intended to investigate the effect of rice bran extract against 3-NP induced motor, behavior, biochemical, neuroinflammation and neurochemical alterations in rats.
2. Material and methods
2.1. Drugs and chemicals
3-NP was procured from Sigma⿿Aldrich, St. Louis, MO, USA. Rice bran was collected from the rice mills (PUSA-44 rice variety) of Moga, Punjab, India. The voucher specimens were deposited in the department of Pharmacognosy at our institute (specimen number HERB/COG/2014-15/1197/69). Interleukin-1 beta (IL-1β), Interleukin-6 (IL-6) and Tumor Necrosis Factor-alpha (TNF-α) Kits were purchased from Krishgen Bio. Sys., Ashley ct Whittier, CA, USA. Unless stated, all other chemicals and biochemical reagents of highest analytical grade were used for the study.
2.2. Extraction procedure
Fresh powder of rice bran was stabilized by sun drying to destroy the enzyme lipase which causes development of free fatty acids [10]. The extracts (hexane and ethanol) of rice bran were prepared using Soxhlation process. Rice bran powder (500 g) was taken and subjected to Soxhlation using hexane as solvent under temperature 60⿿80 °C. Further, the hexane extract was concentrated under vacuum using rotary evaporator (Euitron Roteva, Media Instrument Mfg. Co., Mumbai). The marc was then subjected to Soxhlation using ethanol as solvent under temperature 70⿿80 °C. The resultant ethanolic extract was concentrated under vacuum using rotary evaporator (Euitron Roteva, Media Instrument Mfg. Co., Mumbai).
2.3. Standardization of rice bran extracts using HPLC
The presence of tocopherols was estimated by HPLC (Waters standard system) consisting of a gradient pump, a 20 μl injector valve, C18 reverse phase column and PDA detector. Methanol⿿water (97:3% v/v) was used as solvent system. Column temperature was maintained at 25 °C. Separation was carried out at a flow rate of 1.5 ml/min [11]. Samples (50 μl) were being injected automatically. Hexane extract was dissolved in methanol and solution was filtered through 0.22 μm nylon filters before injecting in the HPLC sample injector. Data were recorded and analyzed with the help of Empower 2 software.
2.4. Experimental animals
Adult male Wistar rats (3⿿4 months old), weighing 250⿿280 g were procured from central animal house of I.S.F. College of Pharmacy, Moga, Punjab (India). The animals were housed in polyacrylic cages in a well controlled atmosphere (room temperature 25 ± 1 °C and relative humidity of 60%) with 12 h light/dark cycle (lights turned on at 7 AM). The animals were maintained on a commercial food diet in the form of dry pellets and water ad libitum. All the behavioral parameters were assessed between 9:00 and 17:00 h. The protocol of the study was approved by the Institutional Animal Ethics Committee (IAEC) (Approval No. IAEC/CPCSEA/M14/P246) and was carried out in accordance with the Indian National Science Academy (INSA) guidelines for the use and care of experimental animals. All the experiments for a given treatment were performed using age-matched animals in an effort to avoid variability between experimental groups.
2.5. Acute oral toxicity test and dose selection
The acute oral toxicity and dose selection of hexane and ethanol extracts were conducted in female albino mice according to Organization for Economic Co-operation and Development (OECD) 425 guidelines (Acute Oral Toxicity ⿿ Up-and-Down Procedure; OECD 425/2001). Animals were fasted 3⿿4 h prior to dosing. Hexane and ethanol extracts were administered to animals in different dose levels up to 5000 mg/kg by oral gavage. Animals were observed daily for 14 days for clinical signs of toxicity including behavioral changes, locomotion and mortality.
2.6. Experimental grouping
The experimental protocol was divided into two phases and consists of six groups. The experimental protocol for the phase I and II study is given below:
Phase I: In this phase, we have evaluated the effect of ethanol and hexane extract at medium dose and effect of both extracts was compared. On the basis of this, we found that hexane extract is better in ameliorating the 3-NP induced neurotoxicity.
Group I: Vehicle treated group (Saline; i.p + CMC; p.o)
Group II: 3-NP (10 mg/kg; i.p) + CMC; p.o
Group III: 3-NP (10 mg/kg; i.p) + Ethanol extract (250 mg/kg; p.o)
Group IV: 3-NP (10 mg/kg; i.p) + Hexane extract (250 mg/kg; p.o)
Phase II: On the basis of results obtained in the phase I, testing of high (500 mg/kg) and low dose (125 mg/kg) of hexane extract was done.
Group V: 3-NP (10 mg/kg; i.p) + Hexane extract (125 mg/kg; p.o)
Group VI: 3-NP (10 mg/kg; i.p) + Hexane extract (500 mg/kg; p.o)
2.7. Treatment schedule
3-NP was dissolved in normal saline (adjusted pH to 7.4) and administered intraperitoneally (i.p) at a dose of 10 mg/kg for 21 days. Ethanol extract (250 mg/kg) and hexane extract (125, 250, 500 mg/kg) were suspended in 0.5% w/v CMC (carboxy methyl cellulose) solution and administered per orally for 21 days in 3-NP treated groups, one and half hour prior to 3-NP dosing. On 0, 7th, 14th, and 21st days, behavioral parameters like body weight, narrow beam, grip strength, locomotor activity and motor coordination were assessed. On 22nd day, animals were sacrificed and striatum was isolated to carry out the evaluation of biochemical [Lipid peroxidation (LPO), Glutathione (GSH), and nitrate], mitochondrial dysfunction (Complex II) and neurochemical [Dopamine (DA), Nor-epinephrine (NE), 5-hydroxy tryptamine (5-HT), GABA, glutamate and their metabolites] parameters. ELISA Kits were used to estimate the levels of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α).
2.8. Measurement of body weight
Animal body weight was recorded on the first and last days (21st day) of experiment [12]. Percentage change in body weight was calculated.
Change in Body Weight = Body Weight (1st day ⿿ 21st day)/Body Weight (1st day) ÿ 100
2.9. Behavioral assessment
2.9.1. Narrow beam walking test
Gait abnormalities and foot slips counts were measured by narrow beam walk apparatus. The animal was made to walk on across a narrow wooden beam. The beam consisted of two platforms (8 cm in diameter) connected by a wooden beam (0.5 mm in thickness, 2.0 cm in width, and 120 cm in length). The beam was elevated 50 cm above ground. A box filled with sawdust was placed below the beam, serving as protection for a falling rat. Rat was allowed to explore on elevated beam for 5 min before training, so that rat get adapt to it. A training trial was then begun by placing a rat on the platform at one end. When a rat walked across the beam from one end to the other end, slipping of its feet occurred. Number of slips and time taken to cross the beam in each trial was recorded [13], [14].
2.9.2. Grip strength measurement
Grip strength of the fore limbs was measured using digital grip force meter (DFIS series, Chatillon, Greensboro, NC, USA). To measure forelimb grip strength, the rat was held by tail and lowered toward the apparatus. The rat was positioned to grab the grid with the fore limbs and was gently pulled back to record the grip strength. The grip strength was recorded in Kgf [15].
2.9.3. Rotarod activity
Motor coordination and grip strength of rats was evaluated using rotarod apparatus (INCO Medicraft, Ambala, India). Before actual recording on rotarod apparatus, the animals were given a prior training session for acclimatization on rotarod (constant speed of 25 rpm; rod diameter 7 cm). The cut off time was 180 s and each rat performed three separate trials after a 5 min gap. The average time of the fall was recorded [16], [17].
2.9.4. Open field test
Open field apparatus consist of wooden, rectangular, light brown colored apparatus consisted of a square (100 ÿ 100 cm) with a surrounding wall (height: 40 cm). It was used to monitor locomotor activity of rats. The floor of the apparatus was divided into 25 small squares. A 40 watt white bulb was lit 150 cm above the test apparatus and the room was illuminated. Before actual recording in open field apparatus, the animals were given a prior 15 min time to move freely for acclimatization. Two hours after the single exposure of apparatus, the animal was placed in the center and number of squares crossed/10 min, number of grooming/10 min and number of rearing/10 min by the animal were recorded. Each crossing was considered only when all the four paws of the animal were in another square. After each trial, apparatus was cleaned properly. Total activity/10 min were calculated by addition of number of squares crossed, number of grooming and number of rearing by the animal [18], [19].
2.10. Dissection and homogenization
On the 21st day, animals from each group were randomly divided into three sub-groups, one for biochemical estimations, second for neuroinflammatory markers estimation and third for neurochemical estimation immediately after the behavioral assessments. The brains were dissected out and placed on ice. Then the striatum was separated and 10% (w/v) tissue homogenate was prepared in 0.1 M phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000 g for 15 min. Aliquots of the supernatant was separated and used for biochemical estimation.
2.11. Measurement of oxidative stress parameters
2.11.1. Measurement of lipid peroxidation
The quantitative measurement of malondialdehyde (end product of lipid peroxidation) in the brain striatum was performed according to the method of [20]. The amount of malondialdehyde (MDA) was measured after its reaction with thiobarbituric acid at 532 nm using spectrophotometer (Shimadzu, UV-1700). The concentration of MDA was expressed as n mol per mg protein.
2.11.2. Estimation of nitrite
The accumulation of nitrite in the striatum supernatant, an indicator of the production of nitric oxide, was determined by a colorimetric assay with Greiss reagent [0.1% N-(1-naphthyl) ethylenediame dihydrochloride, 1% sulphanilamide and 2.5% phosphoric acid] as described by [21]. Absorbance was measured at 540 nm using spectrophotometer (UV-1700, Shimadzu, Japan). The concentration of nitrite was expressed as μmol per mg protein.
2.11.3. Estimation of glutathione
The level of reduced glutathione in striatum of brain was estimated according to the described method [22]. Absorbance was measured at 412 nm using spectrophotometer (Shimadzu, UV-1700). The concentration of glutathione was expressed as μmol per mg protein.
2.11.4. Protein estimation
The protein was measured by the Lowry method using Folin phenol reagent [23]. The phenolic group of tyrosine and trytophan residues (amino acid) in a protein will produce a blue purple color complex with Folin⿿Ciocalteu reagent which consists of sodium tungstate molybdate and phosphate. Absorbance was measured at 660 nm using spectrophotometer (Shimadzu, UV-1700).
2.12. Mitochondrial complex estimation
2.12.1. Isolation of rat brain mitochondria
Rat brain mitochondria were isolated by the described method [24]. The brain striatum was homogenized in isolation buffer with EGTA (215 mM mannitol, 75 mM sucrose, 0.1% Bovine serum albumin (BSA), 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1 mM Ethylene glycol tetraacetic acid (EGTA), pH 7.2). The homogenate was centrifuged at 13,000 g for 5 min at 4 °C. The pellet was re-suspended in isolation buffer with EGTA and spun again at 13,000 g for 5 min. The resulting supernatant was transferred to new tubes and topped off with isolation buffer containing EGTA and spun again at 13,000 g for 10 min. The pellet containing pure mitochondria was re-suspended in isolation buffer without EGTA.
2.12.2. Complex II (SDH activity)
Succinate dehydrogenase (SDH) was measured spectrophotometrically according to the described method by [25]. The method involves the oxidation of succinate by an artificial electron acceptor, potassium ferricyanide. The reaction mixture contained 0.2 M phosphate buffer pH 7.8, 1% BSA, 0.6 M succinic acid and 0.03 M potassium ferricyanide. The reaction was initiated by addition of the striatum mitochondrial sample, and the absorbance change at 420 nm was followed for 2 min (protein concentration was 0.67 mg/ml).
2.13. Estimation of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) levels
The quantifications of IL-1β, IL-6 and TNF-α were done by using rat IL-1β, IL-6 and TNF-α immunoassay kit (KRISHGEN BioSystems, Ashley ct Whittier, CA, USA). The quantikine rat IL-1β, IL-6 and TNF-α immunoassay is a 4.5 h solid phase ELISA designed to measure IL-1β, IL-6 and TNF-α levels. It is a solid-phase sandwich enzyme linked immunosorbent assay (ELISA) using a microtitre plate reader. Concentrations of proinflammatory cytokines were calculated from the standard curves.
2.14. Neurochemical estimation
2.14.1. Estimation of catecholamines
Catecholamines (DA, 5-HT and NE) and their metabolites [3,4-dihydro phenyl acetaldehyde (DOPAC), 5-hydroxyindoleacetic acid (5-HIAA), homovanillic acid (HVA)] levels were estimated by HPLC using electrochemical detector. Waters standard system consisting of a high pressure isocratic pump, a 20 μl manual injector valve, C18 reverse phase column and electrochemical detector were used in the study. Mobile phase consisted of sodium citrate buffer (pH 4.5) ⿿ acetonitrile (87:13), v/v). Sodium citrate buffer consisted of 10 mM citric acid, 25 mM NaH2PO4, 25 mM ethylenediaminetetraacetic acid (EDTA), and 2 mM of 1-heptane sulfonic acid. Electrochemical conditions for the experiment were +0.75 V, sensitivity ranged from 5 to 50 nA. Separation was carried out at a flow rate of 0.8 ml/min. Samples (20 μl) were injected manually. On the day of experiment frozen brain samples were thawed and they were homogenized in homogenizing solution containing 0.2 M perchloric acid. After that samples were centrifuged at 12,000 g for 5 min. The supernatant was filtered through 0.22 μm nylon filters before injecting in the High performance liquid chromatography (HPLC) sample injector [26]. Data were recorded and analyzed with the help of breeze software. Concentrations of neurotransmitter and their metabolites were calculated from the standard curve generated by using standard in a concentration range of 10⿿100 ng/ml.
2.14.2. Estimation of GABA and glutamate
The estimation of GABA and glutamate was done by method described by Donzanti and Yamamoto [27] with slight modifications. The mobile phase was comprised of 100 mM disodium hydrogen phosphate anhydrous, 25 mM EDTA and 22% methanol (pH 6.5). Electrochemical conditions for the experiment were +0.65 V, sensitivity ranges from 5 to 50 nA. Separation was carried out at a flow rate of 1.2 ml/min and column temperature was maintained at 40 °C. Samples (20 μl) were injected manually through rheodyne valve injector. On the day of experiment frozen brain samples were thawed and homogenized in 0.2 M perchloric acid. After that samples were centrifuged at 12,000 g for 15 min. The supernatant was derivatized using OPA/β-ME and then filtered through 0.22 mm nylon filters before injecting into the HPLC sample injector. Data were recorded and analyzed with the help of breeze software. Concentrations of amino acids were calculated from the standard curve generated by using standard in a concentration range of 10⿿100 ng/ml. The values are expressed as percentage of Normal Control group.
2.14.3. Pre-column derivatisation procedure
Amino acids were measured as OPA/β-ME derivatives according to the method of Donzanti and Yamamoto [27]. Stock solution of amino acids standards were prepared at the level of 1 mg/ml. Stock solution of OPA/β-ME derivatizing reagent were prepared by dissolving 27 mg OPA in 1 ml methanol. 5 μl of β-ME and 9 ml tetraborate buffer (0.1 M Sodium tetraborate, pH 10.3) were then added. This solution is stable upto 5 days when protected from light. The working OPA/β-ME derivatizing reagent was prepared by diluting 2.5 ml of stock OPA/βME solution with 7.5 ml of tetraborate buffer. This solution must be prepared fresh daily. Brain samples were derivatized by mixing sample and reagent in ratio of 1:1.5 (sample:reagent ratio).
3. Statistical analysis
The results are expressed as mean ± SD. The behavioral data were analyzed using two way analysis of variance (ANOVA) followed by Bonferroni post hoc test for multiple comparison. p < 0.005 was considered statistically significant. For biochemical parameters, one way analysis of variance (ANOVA) followed by Tukey's post hoc test was used for comparison. p < 0.05 was considered statistically significant.
4. Results
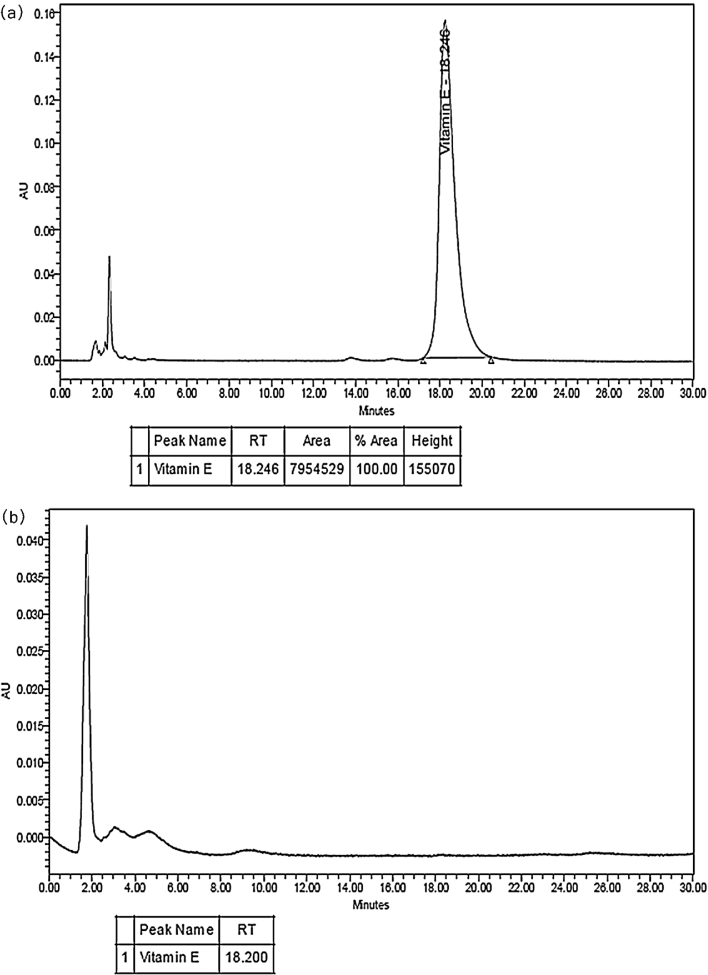
HPLC results of both extracts revealed the presence of tocopherols (vitamin E) in hexane extract and absence of tocopherols in ethanol extract.
Hexane and ethanol extracts of rice bran were prepared and tested in 3-NP treated rats at dose of 250 mg/kg; p.o. In present study, firstly we compared the results of hexane and ethanol extract and found that hexane extract showed more promising effects as compared to ethanol extract. Based on these findings we further preceded with lower (125 mg/kg; p.o) and higher doses (500 mg/kg; p.o) of hexane extract and checked their activity in 3-NP treated rats.
4.1. HPLC analysis of hexane extract and ethanol extract for presence of tocopherols
HPLC of both extracts was performed to check the presence of tocopherols using PDA detector (Fig. 1a and b). Only hexane extract showed the presence of tocopherols.
Fig. 1.
Estimation of tocopherols presence in hexane extract (a) and ethanol extract (b) using HPLC-PDA detector.
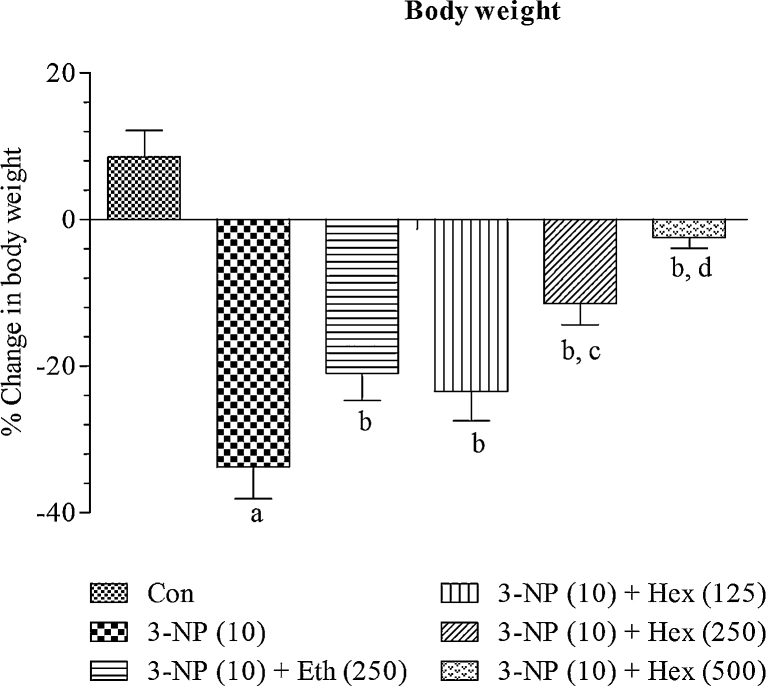
4.2. Effect of rice bran extracts on 3-NP induced decrease in body weight
There was no significant change in initial and final body weight of normal control animals. 3-NP treatment (10 mg/kg/day; i.p) caused a significant (p < 0.001) gradual decrease in body weight as compared to normal control group indicating development of neurotoxicity. Pretreatment with hexane (250 mg/kg/day; p.o) and ethanol extract (250 mg/kg/day; p.o) significantly (p < 0.001) attenuated the fall in body weight as compared to 3-NP treated group.
Hexane extract (250 mg/kg/day; p.o) showed more significant (p < 0.05) effect as compared to ethanol extract (250 mg/kg/day; p.o). On the basis of these results, lower (125 mg/kg/day; p.o) and higher (500 mg/kg/day; p.o) doses of hexane extract were tested in 3-NP treated rats. Hexane extract (125 and 500 mg/kg/day; p.o) significantly (p < 0.05) prevented fall in body weight in 3-NP treated rats. The effect of hexane extract (500 mg/kg/day; p.o) was more significant (p < 0.05) as compared to hexane extract (250 mg/kg/day; p.o) (Fig. 2).
Fig. 2.
Effect of rice bran extracts on 3-NP induced decrease in body weight: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
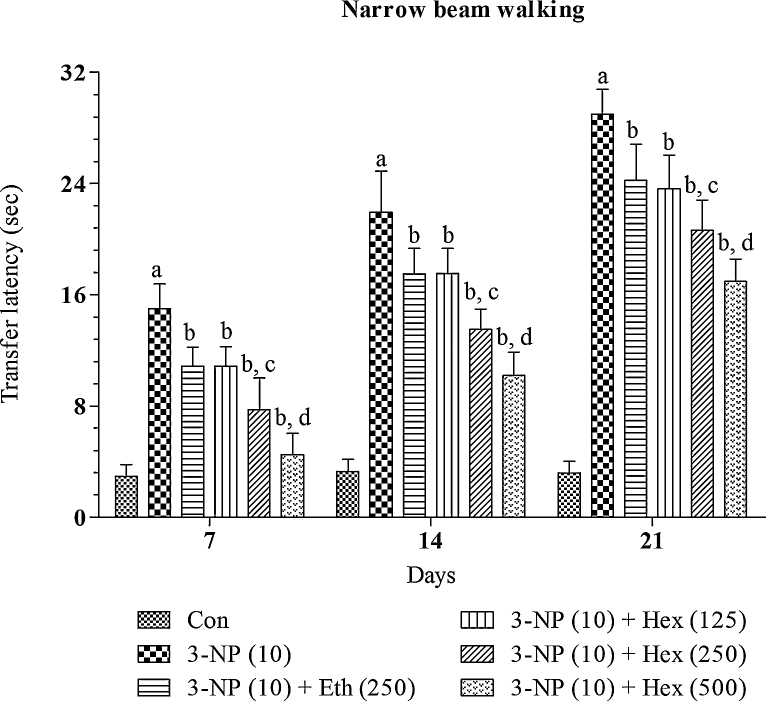
4.3. Effect of rice bran extracts on narrow beam walk performance in 3-NP treated rats
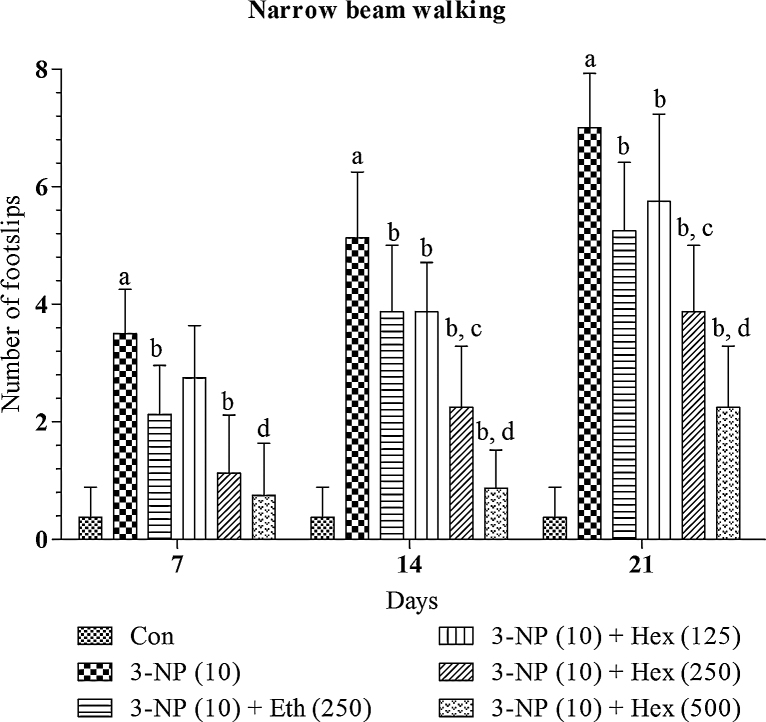
Systemic 3-NP (10 mg/kg/day; i.p) treatment produced significant (p < 0.01) gradual increase in transfer latency to cross narrow beam runway and foot slips on narrow beam walk as compared to normal control group (Fig. 3, Fig. 4). Whereas, 3-NP administered rats pretreated with hexane (250 mg/kg/day; p.o) and ethanol extract (250 mg/kg/day; p.o) lead to significant (p < 0.05) decrease in transfer latency to cross beam and foot slips on narrow beam walk as compared to 3-NP alone treated rats.
Fig. 3.
Effect of rice bran extracts on narrow beam walk transfer latency in 3-NP treated rats: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
Fig. 4.
Effect of rice bran extracts on number of foot slips on narrow beam walk apparatus in 3-NP treated rats: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
Further, hexane extract (125 and 500 mg/kg/day; p.o) significantly (p < 0.05) prevented increase in transfer latency to cross beam and foot slips on narrow beam walk in 3-NP treated rats. Hexane extract produced dose dependent effects. The effect of hexane extract (500 mg/kg/day; p.o) was more significant (p < 0.05) as compared to hexane extract (250 mg/kg/day; p.o).
4.4. Effect of rice bran extracts on 3-NP induced changes in grip strength performance, rotarod activity and locomotor activity
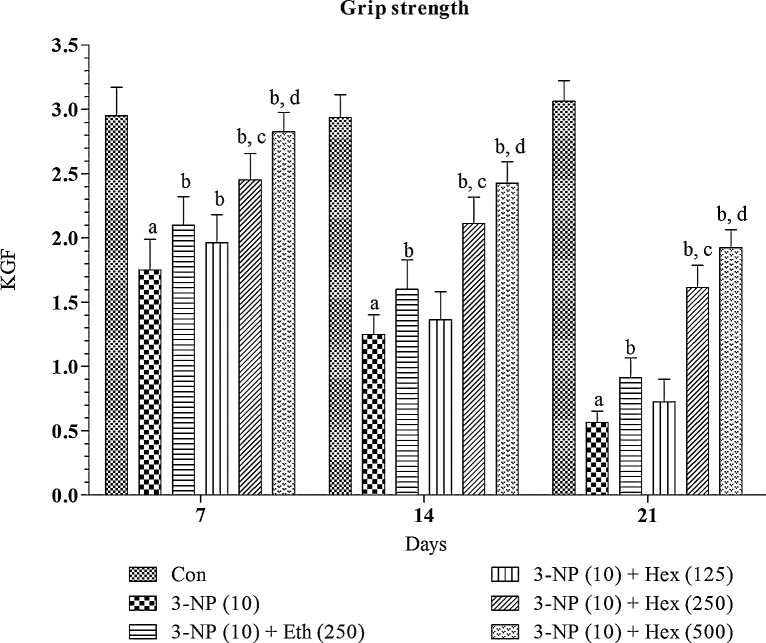
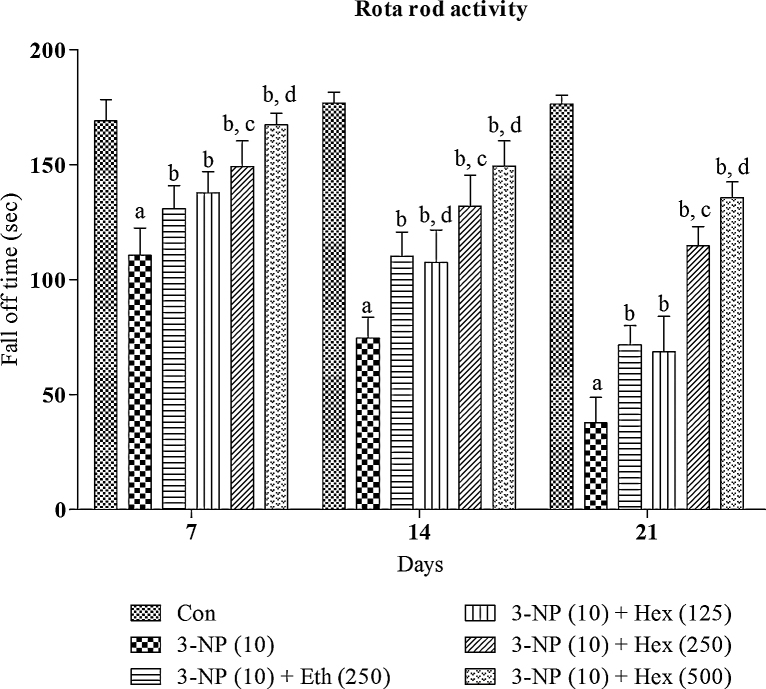
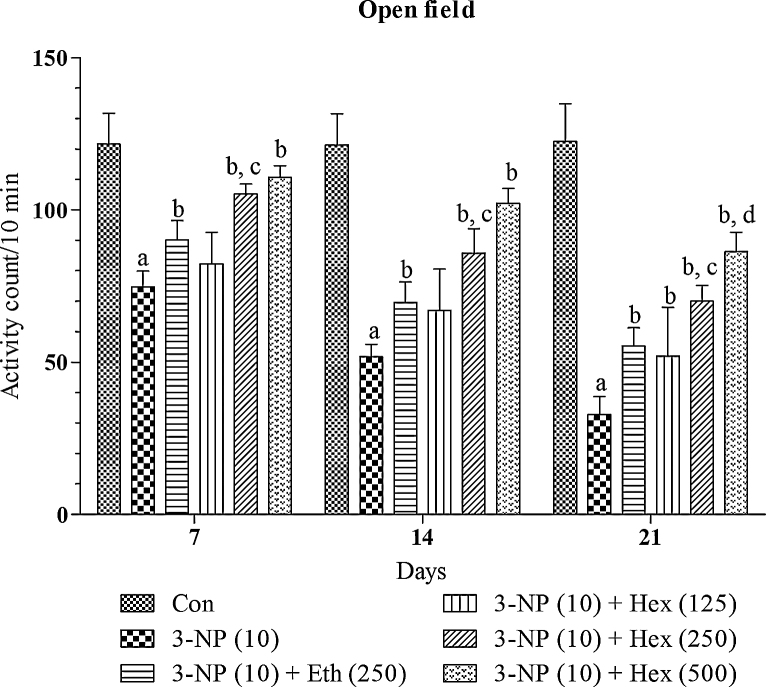
Systemic 3-NP (10 mg/kg/day; i.p) treatment produced significant (p < 0.001) gradual decrease in grip strength, rotarod activity and locomotor activity on 2nd (day 14th) and 3rd week (day 21st) as compared to normal control group (Fig. 5, Fig. 6, Fig. 7). Pretreatment with hexane extract (250 mg/kg/day; p.o) and ethanol extract (250 mg/kg/day; p.o) significantly (p < 0.05) prevented the impairment in grip strength, rotarod activity and locomotor activity as compared to 3-NP treated rats.
Fig. 5.
Effect of rice bran extracts on 3-NP induced changes in grip strength performance: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
Fig. 6.
Effect of rice bran extracts on 3-NP induced changes in rotarod activity: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
Fig. 7.
Effect of rice bran extracts on 3-NP induced changes in locomotor activity: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
Hexane extract (125 mg/kg/day; p.o) showed no significant restoration of grip strength activity in 3-NP treated rats as compared to 3-NP alone treated rats whereas it showed significant (p < 0.05) restoration of rotarod and locomotion activity in 3-NP treated rats as compared 3-NP alone treated rats. On the other side, hexane extract (500 mg/kg/day; p.o) significantly (p < 0.001) prevented decrease in grip strength, rotarod activity and locomotion activity in 3-NP treated rats as compared 3-NP alone treated rats. The effect of hexane extract (500 mg/kg/day; p.o) was more significant (p < 0.05) as compared to hexane extract (250 mg/kg/day; p.o)
4.5. Effect of rice bran extracts on lipid peroxidation, nitrite and glutathione levels in 3-NP administered rats
Systemic administration of 3-NP (10 mg/kg/day; i.p) significantly (p < 0.001) increased lipid peroxidation, nitrite concentration and depleted glutathione enzyme activity in the striatum as compared to normal control group (Table 1). Pretreatment with hexane extract (250 mg/kg/day; p.o) and ethanol extract (250 mg/kg/day; p.o) significantly (p < 0.05) attenuated lipid peroxidation, nitrite concentration and restored levels of antioxidant enzyme glutathione as compared to 3-NP alone treated group.
Table 1.
Effect of rice bran extracts on lipid peroxidation, glutathione and nitrite levels in 3-NP administered rats. Data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
| Treatment (mg/kg) | MDA (n moles of MDA/mg protein) | GSH (μ moles of GSH/mg protein) | Nitrite (μg/ml) |
|---|---|---|---|
| Con | 0.216 ± 0.136 | 0.0324 ± 0.0062 | 72.17 ± 23.57 |
| 3-NP (10) | 1.748 ± 0.108 a | 0.0011 ± 0.0008 a | 389.0 ± 47.25 a |
| Eth (250) | 1.221 ± 0.220 b | 0.0091 ± 0.0020 b | 320.8 ± 30.52 b |
| Hex (250) | 0.781 ± 0.184 b,c | 0.0180 ± 0.0052 b,c | 182.8 ± 29.61 b,c |
| Hex (125) | 1.353 ± 0.366 b | 0.0088 ± 0.0013 | 356.0 ± 37.35 |
| Hex (500) | 0.402 ± 0.122 b,d | 0.0273 ± 0.0057 b,d | 106.3 ± 26.17 b,d |
After that, hexane extract (125 mg/kg/day; p.o) showed no significant effect in preventing increase in nitrite concentration whereas it significantly (p < 0.05) attenuated lipid peroxidation and showed significant (p < 0.05) restoration of glutathione levels as compared to 3-NP alone treated group. On the other hand, hexane extract (500 mg/kg/day; p.o) significantly (p < 0.05) attenuated lipid peroxidation, nitrite concentration and restored levels of antioxidant enzyme glutathione as compared to 3-NP alone treated group. Effects produced by hexane extract (500 mg/kg/day; p.o) in decreasing lipid peroxidation and nitrite concentration and restoring glutathione levels were more significant (p < 0.05) as compared to hexane extract (250 mg/kg/day; p.o).
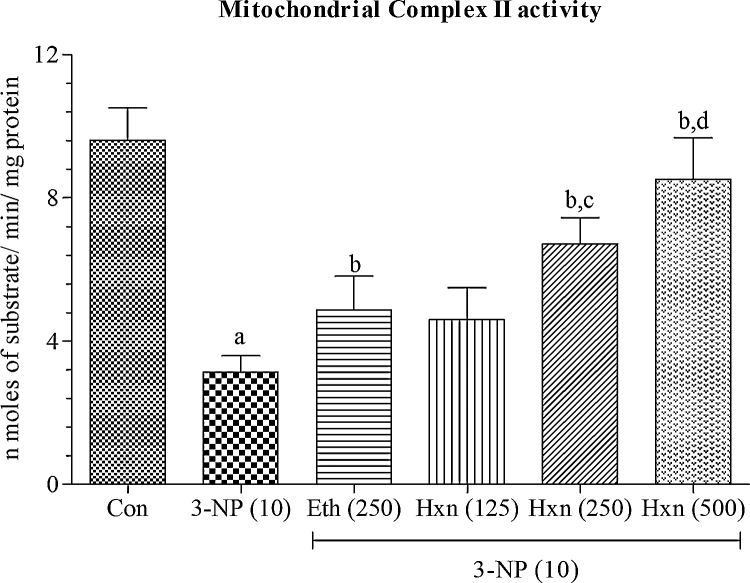
4.6. Effect of rice bran extracts on mitochondrial enzyme complex II levels in 3-NP treated rats
Systemic administration of 3-NP (10 mg/kg/day; i.p) significantly (p < 0.001) impaired the mitochondrial enzyme complex II activity as compared to normal control group (Fig. 8). Pretreatment with hexane extract (250 mg/kg/day; p.o) and ethanol extract (250 mg/kg/day; p.o) significantly (p < 0.05) restored the mitochondrial enzyme complex II activity as compared to 3-NP treated group.
Fig. 8.
Effect of rice bran extracts on mitochondrial enzyme complex II levels in 3-NP treated rats: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
Hexane extract (125 mg/kg/day; p.o) showed no significant effect in restoration of mitochondrial enzyme complex II activity as compared to 3-NP alone treated group whereas, hexane extract (500 mg/kg/day; p.o) significantly (p < 0.05) and dose dependently prevented impairment of mitochondrial complex II activity as compared to 3-NP alone treated group and hexane extract (250 mg/kg; p.o) treated group.
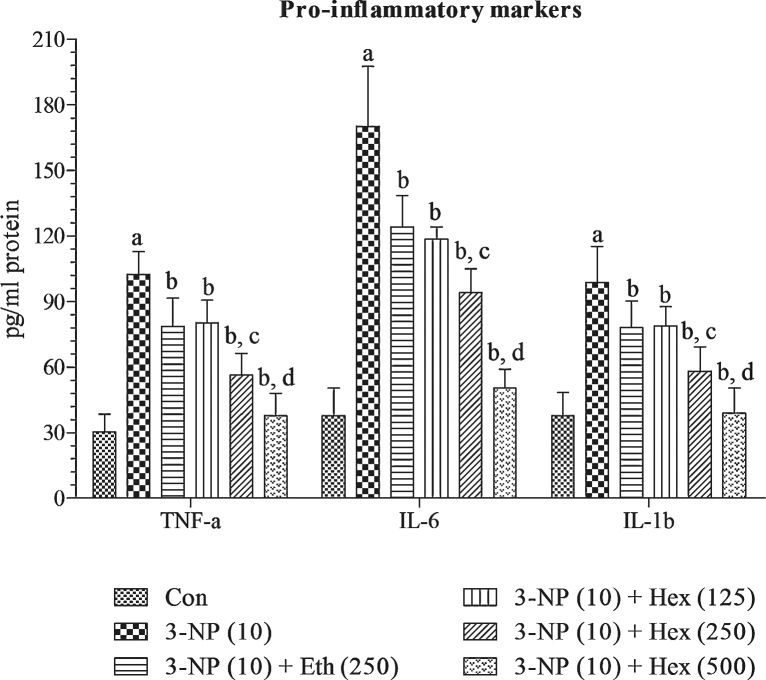
4.7. Effect of rice bran extracts on pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in 3-NP treated rats
Systemic 3-NP (10 mg/kg/day; i.p) treatment produced significant (p < 0.001) elevation in striatal IL-1β, IL-6 and TNF-α levels as compared to normal control group. Pretreatment with hexane extract (250 mg/kg/day; p.o) and ethanol extract (250 mg/kg/day; p.o) significantly (p < 0.05) attenuated the levels of IL-1β, IL-6 and TNF-α in striatum as compared to 3-NP alone treated group. Hexane extract (250 mg/kg/day; p.o) showed more significant (p < 0.05) and marked effects in attenuating the levels of IL-1β, IL-6 and TNF-α in striatum as compared to ethanol extract (250 mg/kg/day; p.o). On the basis of these results, lower (125 mg/kg/day; p.o) and higher (500 mg/kg/day; p.o) doses of hexane extract were tested in 3-NP treated rats. Hexane extract (125 mg/kg/day; p.o) and hexane extract (500 mg/kg/day; p.o) significantly (p < 0.05) and dose dependently prevented increase in the levels of IL-1β, IL-6 and TNF-α as compared to 3-NP alone treated group (Fig. 9).
Fig. 9.
Effect of rice bran extracts on pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in 3-NP treated rats: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
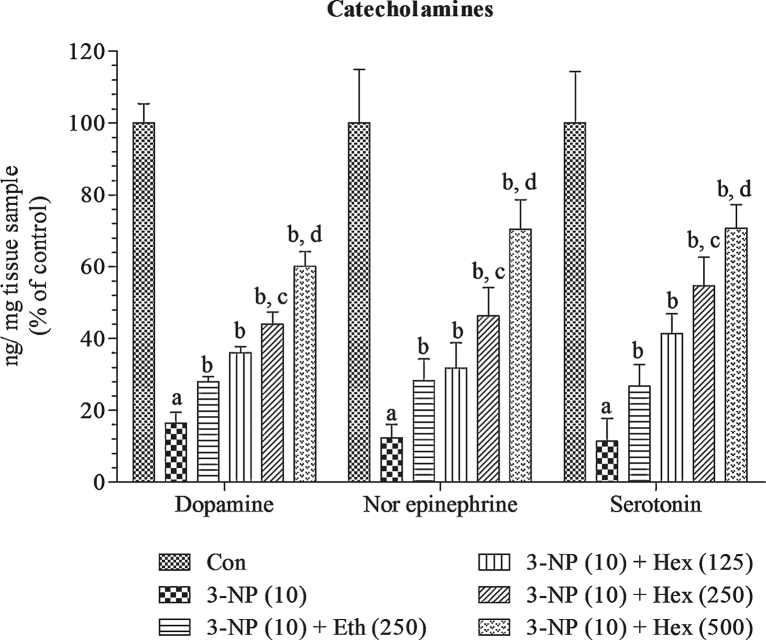
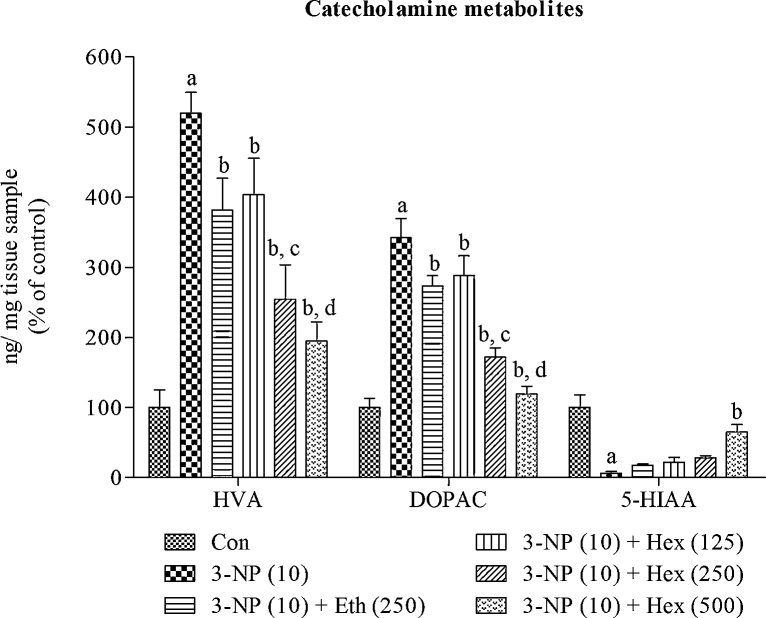
4.8. Effect of rice bran extracts on brain catecholamines and their metabolites levels in 3-NP treated rats
Systemic 3-NP (10 mg/kg/day; i.p) treatment caused significant (p < 0.001) decrease in levels of catecholamines (NE, DA and 5-HT) and 5-HIAA in striatum and increase in levels of DOPAC and HVA as compared to normal control group. Pretreatment with hexane extract (250 mg/kg/day; p.o) and ethanol extract (250 mg/kg/day; p.o) significantly (p < 0.05) increased the levels of NE, DA, 5-HT and 5-HIAA and decreased the levels of DOPAC and HVA as compared to 3-NP alone treated group.
Hexane extract (125 and 500 mg/kg/day; p.o) significantly (p < 0.05) prevented decrease in levels of NE, DA, 5-HT and 5-HIAA and increase in levels of DOPAC and HVA as compared to 3-NP alone treated group. At last, hexane extract (500 mg/kg/day; p.o) showed more significant (p < 0.05) effect by attenuating decrease in levels of NE, DA, 5-HT and 5-HIAA and increase in levels of DOPAC and HVA as compared to hexane extract (250 mg/kg/day; p.o) (Fig. 10, Fig. 11).
Fig. 10.
Effect of rice bran extracts on brain catecholamines levels in 3-NP treated rats: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
Fig. 11.
Effect of rice bran extracts on brain catecholamines metabolites levels in 3-NP treated rats: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
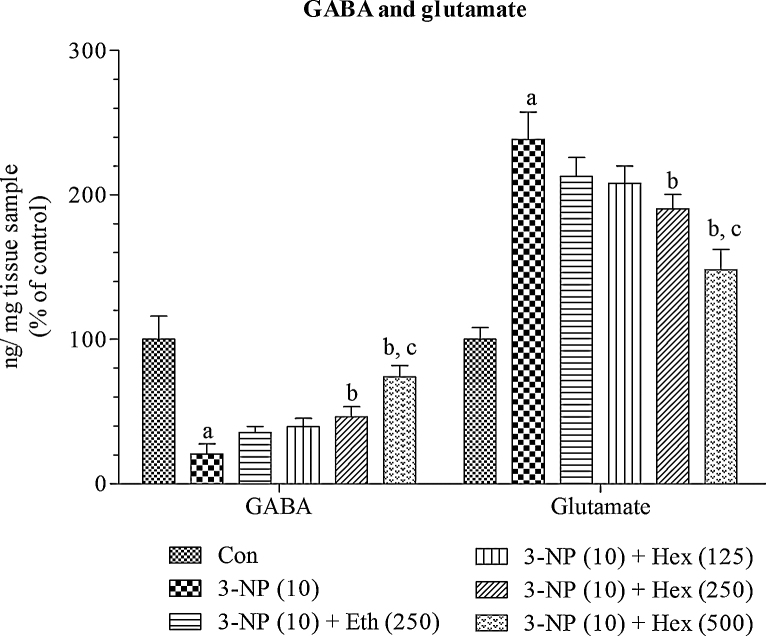
4.9. Effect of rice bran extracts on brain GABA and Glutamate levels in 3-NP treated rats
Systemic 3-NP (10 mg/kg/day; i.p) treatment caused significant (p < 0.001) decrease in levels of GABA in striatum and increase in levels glutamate as compared to normal control group. Pretreatment with hexane extract (250 mg/kg/day; p.o) significantly (p < 0.05) increased the levels of GABA and decreased the levels of glutamate, whereas pretreatment with ethanol extract (250 mg/kg/day; p.o) did not show any significant effect on levels of GABA and glutamate as compared to 3-NP alone treated group.
Hexane extract (500 mg/kg/day; p.o) significantly (p < 0.05) prevented decrease in levels of GABA and increase in levels of, whereas hexane extract (125 mg/kg; p.o) did not show any significant effect on levels of GABA and glutamate as compared to 3-NP alone treated group. At last, hexane extract (500 mg/kg/day; p.o) showed more significant (p < 0.05) effect by attenuating decrease in levels of GABA and increase in levels of glutamate as compared to hexane extract (250 mg/kg/day; p.o) (Fig. 12).
Fig. 12.
Effect of rice bran extracts on brain GABA and Glutamate levels in 3-NP treated rats: data expressed as mean ± SD: pa < 0.001 v/s Con, pb < 0.05 v/s 3-NP (10), pc < 0.05 v/s Eth (250), pd < 0.05 v/s Hex (250). Con = normal control; 3-NP = 3-nitropropionic acid; Hex = hexane; Eth = ethanol.
5. Discussion
In this study, we have investigated the role of rice bran extract against 3-NP induced neurotoxicity. Rice bran extract is the by-product of rice milling process. It contains a variety of components including γ-oryzanol, coumaric acid, ferulic acid, caffeic acid, phytic acid; carotenoids such as α- and β-carotene, lutein, and lycopene, zeazanthin; the vitamin E isoforms α, β, γ and δ-tocopherol, and tocotrienols; phytosterols such as b-sitosterol, campesterol and stigmasterol [28]. Rice bran also contains pectin, cellulose, hemicelluloses, arabinoxylin; essential amino acids like tryptophan, arginine, histidine, cysteine and methionine; and micronutrients such as phosphorous, magnesium, manganese, calcium and 9 B-vitamins [29]. Recently, rice bran has gained much attention, as numerous studies have revealed the therapeutic potential against oxidative stress, cancer, type II diabetes, inflammation, hyperlipidemia, coronary heart disease, Alzheimer's disease and aging [10], [30]. Hexane and ethanol extracts of rice bran were prepared using Soxhlation and activity of both the extracts was tested at dose of 250 mg/kg in 3-NP treated rats. Hexane extract showed significant and beneficial effects as compared to ethanol extract at 250 mg/kg dose. Based on above findings, we further proceeded with lower (125 mg/kg) and higher (500 mg/kg) dose of 3-NP in 3-NP treated group.
3-NP is a mitochondrial toxin which irreversibly inhibits the mitochondrial complex II enzyme (succinate dehydrogenase) and acts as a gold standard model for induction of HD like symptoms in rats. It has been reported that systemic administration of 3-NP imitate all the pathophysiological features of HD like mitochondrial dysfunction, excitotoxicity, oxidative stress as seen clinically and produced selective striatal degeneration leading to neurochemical imbalance [31], [32]. In present study, 3-NP was administered daily for 21 days and produced significant alteration in body weight, grip strength, motor activity, biochemical parameters, mitochondrial enzyme complex II function, pro-inflammatory cytokines level and neurochemicals in rats. This impairment in locomotor activity, motor functions and grip strength was due to selective degeneration of GABAergic MSNs of striatum. Another trait of 3-NP induced neurotoxicity is decrease in body weight, as observed in present study, which might be due to energy defects and decrease in ATP generation in the body because of mitochondrial complex II inhibition. The present findings are in harmony with earlier findings following 3-NP administration [33], [34], [31], [35], [36], [12], [32]. Pretreatment with hexane extract significantly prevented the alteration in body weight, grip strength, motor activity in the 3-NP treated animals. This beneficial effect may be attributed to ability of hexane extract to prevent the alteration in the levels of catecholamines in striatum [9].
Oxidative stress is considered as one of the chief determinants of 3-NP induced neurotoxicity as evidenced by increased oxidative damage and diminution of endogenous antioxidant levels [34], [17], [15]. In the present study, 3-NP significantly increased the levels of MDA, nitrite and decreased the levels of glutathione antioxidant enzyme in the striatum. Reports from literature indicate that 3-NP toxicity leads to depletion of ATP and energy failure, altered calcium homeostasis, excitotoxic events, increased reactive oxygen species (ROS) production. ROS production in turn leads to imbalance between oxidants/antioxidants, causing molecular damage that leads to neuronal cell death [6]. In the present study, pre-treatment with hexane extract significantly reversed the 3-NP induced oxidative stress. Reports have shown that hexane extract of rice bran contains tocopherols and tocotrienols, well known antioxidants, contributing to the beneficial effects of this extract against oxidative stress [37], [38]. HPLC results from our lab also proved that hexane extract contains tocopherols and tocotrienols.
Mitochondria are membrane bound organelle, also known as ⿿cellular power plants⿿ because they generate most of the cell's supply of ATP. In addition to supplying cellular energy, mitochondria are involved in signaling, cellular differentiation, cell death, control of the cell cycle and cell growth. In the present study, 3-NP irreversibly inhibited the enzyme succinate dehydrogenase, which is a member of the Krebs tricarboxylic acid cycle (oxidizing succinate to fumarate). This inhibition in turn is associated with generation of ROS. These findings are analogous to previous reports [31], [12], [39]. Pretreatment with hexane extract of rice bran at higher dose significantly prevented the 3-NP induced alteration in mitochondrial enzyme complex II activity in striatum suggesting protective role of hexane extract against mitochondrial dysfunction. Also, the hexane extract of rice bran have been reported to have beneficial effect against mitochondrial dysfunction in Alzheimer disease in previous studies. According to previous reports, hexane extract improved mitochondrial function by increasing mitochondrial membrane potential, ATP levels, complex I and II activity [40], [30].
Neuroinflammation is increasingly being known as a biological process that resembles pathological cascades underlying HD in a very close manner. Pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α have been implicated in the pathogenesis of various neurodegenerative diseases [41]. In the current study, 3-NP lead to significant increase in the levels of IL-1β, IL-6 and TNF-α in striatum as compared to normal control group, as documented in previous studies [15]. Previous literature suggests that in HD, neuroinflammation mediated by cytokines and nitric oxide (NO) finally contributes to excitotoxicity, mitochondrial dysfunction and neurodegeneration [42]. Pretreatment with hexane extract significantly prevented this increase in levels of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α). Previous reports from literature showed that tocopherols, tocotrienols and γ-oryzanol present in rice bran oil have protective effect against neuroinflammation and have free radical scavenging property [43].
Neurochemical imbalance is well reported to occur in HD. In the present study 3-NP administration caused significant decrease in the levels of catecholamines (NE, DA and 5-HT), 5-HIAA and GABA and increase in the levels of DOPAC, HVA and glutamate in the striatum as compared to normal control group. The results are in tune with the previous reports [3]. In HD, previous findings suggest that biphasic changes in dopamine neurotransmission occur. In the early stages the neurotransmission of DA increases and leads to hyperkinesia which can be controlled by depletion of dopamine stores but on the other hand, neurotransmission of DA decreases and lead to hypokinesia in later stages of disease which can be controlled by increasing DA function [48]. The levels of serotonin (5-HT) and its metabolite (5-HIAA) are also found to be decreased in HD [47]. In the striatum, 90% of neurons are medium spiny neurons; GABA-containing projection neurons are preferentially lost in HD [44]. The loss of striatal GABA receptors probably represents the loss of striatal neurons. Pretreatment with hexane extract significantly corrected this imbalance in levels of catecholamines (NE, DA, 5-HT), their metabolites (HVA, DOPAC, 5-HIAA), GABA and glutamate. Reports from previous studies showed that hexane extract of rice bran containing γ-oryzanol, tocopherols and tocotrienols normalizes the level of striatal neurotransmitters (NE, DA and 5-HT) [9]. Previous reports have also elucidated the effects of vitamin E in protecting astrocytes against glutamate injury [45]. Another report form literature have concluded that production of GABA by Lactobacillus sakei B2-16 increases in the presence of rice bran extract [46].
Rice bran is one of the co-products of rice milling process. In the last few years, it has been emerging as a therapeutic agent in various disease conditions. Reports show that rice bran is a potent antioxidant. It contains many natural antioxidants like tocopherols, tocotrienols, oryzanols and ferulic acid esters [10], [37]. Reports from the literature suggest that rice bran protective affect against complex I and complex II of mitochondria. It has been reported that rice bran improves the mitochondrial activity in cellular model of early Alzheimer's disease [40], [30]. Rice bran components like containing γ-oryzanol, tocopherols and tocotrienols are well reported to increase the levels of catecholamines (NE, DA and 5-HT) [9]. Moreover, rice bran is reported to enhance the production of GABA by Lactobacillus sakei B2-16 [46]. Rice bran contains tocopherols and tocotrienols which are protective against excitotoxicity. In the study conducted by Selvaraju and co-workers, the effects of vitamin E (TRF and α-TCP) in protecting astrocytes against glutamate injury were elucidated. Hence, vitamin E acted as a potent antioxidant agent in recovering mitochondrial injury due to elevated oxidative stress, and enhanced better survivability upon glutamate toxicity [45]. Hence, normalization of the level of striatal neurotransmitters can be considered as key factor contributing to the beneficial effect of rice bran. Rice bran constituents like tocopherols, tocotrienols and oryzanols have been reported to exhibit protective effect against neuroinflammation and moreover, act as free radical scavengers also [43].
6. Conclusion
Results obtained from the current study suggested that hexane extract of rice bran showed more beneficial effect as compared to ethanol extract and out of three different doses (125, 250 and 500 mg/kg) of hexane extract, higher dose (500 mg/kg) produced more pronounced effect in ameliorating the 3-NP induced neurotoxicity in rats. The neuroprotective efficacy of hexane extract can be attributed to presence of tocopherols, tocotrienols and γ-oryzanols which have protective effect against mitochondrial dysfunction, oxidative stress, excitotoxicity, neuroinflammation and neurochemical alteration. The outcomes of the present study suggest that hexane extract of rice bran extract is beneficial and therefore might emerge as an adjuvant or prophylactic therapy for treatment of HD like symptoms.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
Authors thankfully acknowledged Science and Engineering Board (SERB), Department of Science and Technology, Govt. of India, New Delhi, for providing financial assistance to Dr. Puneet Kumar under Fast Track Scheme (DST-SERB-FTYS) (SB/FT/LS-139/2012). Authors are also grateful to Mr. Praveen Garg (Chairman, I.S.F College of Pharmacy, Moga) for providing necessary resources and facilities for research work.
References
- 1.Beal M.F., Ferrante R., Swartz K., Kowall N. Chronic quinolinic acid lesions in rats closely resemble Huntington's disease. J. Neurosci. 1991;11(6):1649–1659. doi: 10.1523/JNEUROSCI.11-06-01649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotkin J.L., Surmeier D.J. Corticostriatal synaptic adaptations in Huntington's disease. Curr. Opin. Neurobiol. 2015;33:53–62. doi: 10.1016/j.conb.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar P., Kalonia H., Kumar A. Huntington's disease: pathogenesis to animal models. Pharmacol. Rep. 2010;62(1):1–14. doi: 10.1016/s1734-1140(10)70238-3. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty J., Nthenge-Ngumbau D., Rajamma U., Mohanakumar K. Melatonin protects against behavioural dysfunctions and dendritic spine damage in 3-nitropropionic acid-induced rat model of Huntington's disease. Behav. Brain Res. 2014;264:91–104. doi: 10.1016/j.bbr.2014.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Chitnis S., Karunapuzha C.A. Tetrabenazine in Huntington's disease chorea. Clin. Med. Ther. 2009;(1):669–681. [Google Scholar]
- 6.Túnez I., Tasset I., Pérez-De La Cruz V., Santamaría A. 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington's disease: past, present and future. Molecules. 2010;15(2):878–916. doi: 10.3390/molecules15020878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna D.M., Tadros M.G., Khalifa A.E. ADIOL protects against 3-NP-induced neurotoxicity in rats: possible impact of its anti-oxidant, anti-inflammatory and anti-apoptotic actions. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;60:36–51. doi: 10.1016/j.pnpbp.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Leegwater-Kim J., Cha J.-H.J. The paradigm of Huntington's disease: therapeutic opportunities in neurodegeneration. NeuroRx. 2004;1(1):128–138. doi: 10.1602/neurorx.1.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicero A., Derosa G. Rice bran and its main components: potential role in the management of coronary risk factors. Curr. Top. Nutraceutical Res. 2005;3(1):29–46. [Google Scholar]
- 10.Prasad M.N. Health benefits of rice bran ⿿ a review. J. Nutr. Food Sci. 2011;1(3) [Google Scholar]
- 11.Nada A., Krishnaiah Y., Zaghloul A.-A., Khattab I. Analysis of vitamin E in commercial cosmetic preparations by HPLC. J. Cosmet. Sci. 2009;61(5):353–365. [PubMed] [Google Scholar]
- 12.Kumar P., Kalonia H., Kumar A. Novel protective mechanisms of antidepressants against 3-nitropropionic acid induced Huntington's-like symptoms: a comparative study. J. Psychopharmacol. 2011;25(10):1399–1411. doi: 10.1177/0269881110364269. [DOI] [PubMed] [Google Scholar]
- 13.Wang X.-M., Gao X., Zhang X.-H., Tu Y.-Y., Jin M.-L., Zhao G.-P., Yu L., Jing N.-H., Li B.-M. The negative cell cycle regulator, Tob (transducer of ErbB-2), is involved in motor skill learning. Biochem. Biophys. Res. Commun. 2006;340(4):1023–1027. doi: 10.1016/j.bbrc.2005.12.125. [DOI] [PubMed] [Google Scholar]
- 14.Kalonia H., Kumar P., Kumar A. Licofelone attenuates quinolinic acid induced Huntington like symptoms: possible behavioral, biochemical and cellular alterations. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(2):607–615. doi: 10.1016/j.pnpbp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Khan A., Jamwal S., Bijjem K., Prakash A., Kumar P. Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3β modulators in 3-nitropropionic acid-induced neurotoxicity in rats. Neuroscience. 2015;287:66–77. doi: 10.1016/j.neuroscience.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni S.K. 1st ed. Vallabh Pub; Delhi: 2008. Handbook of Experimental Pharmacology Pract. Pharmacol. Clin. Pharmacy; pp. 136–137. [Google Scholar]
- 17.Kumar P., Kumar P., Khan A., Deshmukh R., Sharma P.L. Role of neurosteroids in experimental 3-nitropropionic acid induced neurotoxicity in rats. Eur. J. Pharmacol. 2014;723:38–45. doi: 10.1016/j.ejphar.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 18.de Lago E., Urbani P., Ramos J.A., Di Marzo V., Fernández-Ruiz J. Arvanil, a hybrid endocannabinoid and vanilloid compound, behaves as an antihyperkinetic agent in a rat model of Huntington's disease. Brain Res. 2005;1050(1):210–216. doi: 10.1016/j.brainres.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Thangarajan S., Deivasigamani A., Natarajan S.S., Krishnan P., Mohanan S.K. Neuroprotective activity of l-theanine on 3-nitropropionic acid-induced neurotoxicity in rat striatum. Int. J. Neurosci. 2014;124(9):673–684. doi: 10.3109/00207454.2013.872642. [DOI] [PubMed] [Google Scholar]
- 20.Wills E. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 1966;99:667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 22.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 24.Berman S.B., Hastings T.G. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria. J. Neurochem. 1999;73(3):1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 25.King T.E. Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Meth. Enzymol. 1967;10:322–331. [Google Scholar]
- 26.Patel B.A., Arundell M., Parker K.H., Yeoman M.S., O⿿Hare D. Simple and rapid determination of serotonin and catecholamines in biological tissue using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci. B. 2005;818(2):269–276. doi: 10.1016/j.jchromb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Donzanti B.A., Yamamoto B.K. An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci. 1988;43(11):913–922. doi: 10.1016/0024-3205(88)90267-6. [DOI] [PubMed] [Google Scholar]
- 28.Henderson A.J., Ollila C.A., Kumar A., Borresen E.C., Raina K., Agarwal R., Ryan E.P. Chemopreventive properties of dietary rice bran: current status and future prospects. Adv. Nutr. 2012;3(5):643–653. doi: 10.3945/an.112.002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan E.P. Bioactive food components and health properties of rice bran. J. Am. Vet. Med. Assoc. 2011;238(5):593–600. doi: 10.2460/javma.238.5.593. [DOI] [PubMed] [Google Scholar]
- 30.Hagl S., Grewal R., Ciobanu I., Helal A., Khayyal M.T., Muller W.E., Eckert G.P. Rice bran extract compensates mitochondrial dysfunction in a cellular model of early Alzheimer's disease. J. Alzheimers Dis. 2015;43(3):927–938. doi: 10.3233/JAD-132084. [DOI] [PubMed] [Google Scholar]
- 31.Brouillet E., Jacquard C., Bizat N., Blum D. 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington's disease. J. Neurochem. 2005;95(6):1521–1540. doi: 10.1111/j.1471-4159.2005.03515.x. [DOI] [PubMed] [Google Scholar]
- 32.Kumar P., Kalonia H., Kumar A. Possible GABAergic mechanism in the neuroprotective effect of gabapentin and lamotrigine against 3-nitropropionic acid induced neurotoxicity. Eur. J. Pharmacol. 2012;674(2):265–274. doi: 10.1016/j.ejphar.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Borlongan C., Koutouzis T., Randall T., Freeman T., Cahill D., Sanberg P. Systemic 3-nitropropionic acid: behavioral deficits and striatal damage in adult rats. Brain Res. Bull. 1995;36(6):549–556. doi: 10.1016/0361-9230(94)00242-s. [DOI] [PubMed] [Google Scholar]
- 34.Kim G.W., Copin J.-C., Kawase M., Chen S.F., Sato S., Gobbel G.T., Chan P.H. Excitotoxicity is required for induction of oxidative stress and apoptosis in mouse striatum by the mitochondrial toxin, 3-nitropropionic acid. J. Cereb. Blood Flow Metab. 2000;20(1):119–129. doi: 10.1097/00004647-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Kumar P., Kumar A. Neuroprotective effect of cyclosporine and FK506 against 3-nitropropionic acid induced cognitive dysfunction and glutathione redox in rat: possible role of nitric oxide. Neurosci. Res. 2009;63(4):302–314. doi: 10.1016/j.neures.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Kumar P., Kumar A. Possible neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of Huntington's disease. J. Med. Food. 2009;12(3):591–600. doi: 10.1089/jmf.2008.0028. [DOI] [PubMed] [Google Scholar]
- 37.Arab F., Alemzadeh I., Maghsoudi V. Determination of antioxidant component and activity of rice bran extract. Sci. Iran. 2011;18(6):1402–1406. [Google Scholar]
- 38.Settharaksa S., Madaka F., Sueree L., Chankana N., Chakree K., Charoenchai L. Cytotoxic activity and antioxidant potentials of cold pressed rice bran oil. Int. J. PharmTech Res. 2014;6(2):686–691. [Google Scholar]
- 39.Sandhir R., Mehrotra A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington's disease. Biochim. Biophys. Acta. 2013;1832(3):421–430. doi: 10.1016/j.bbadis.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Hagl S., Kocher A., Schiborr C., Eckert S.H., Ciobanu I., Birringer M., El-Askary H., Helal A., Khayyal M.T., Frank J. Rice bran extract protects from mitochondrial dysfunction in guinea pig brains. Pharmacol. Res. 2013;76:17–27. doi: 10.1016/j.phrs.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Meffert M.K., Baltimore D. Physiological functions for brain NF-κB. Trends Neurosci. 2005;28(1):37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Ellrichmann G., Reick C., Saft C., Linker R.A. The role of the immune system in Huntington's disease. Clin. Dev. Immunol. 2013:2013. doi: 10.1155/2013/541259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh U., Devaraj S., Jialal I. Vitamin E, oxidative stress, and inflammation. Annu. Rev. Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 44.Faull R., Waldvogel H., Nicholson L., Synek B. The distribution of GABA A-benzodiazepine receptors in the basal ganglia in Huntington's disease and in the quinolinic acid-lesioned rat. Prog. Brain Res. 1993;99:105–123. doi: 10.1016/s0079-6123(08)61341-2. [DOI] [PubMed] [Google Scholar]
- 45.Selvaraju T.R., Khaza⿿ai H., Vidyadaran S., Mutalib M.S.A., Vasudevan R. The neuroprotective effects of tocotrienol rich fraction and alpha tocopherol against glutamate injury in astrocytes. Bosn. J. Basic Med. Sci. 2014;14(4):195–204. doi: 10.17305/bjbms.2014.4.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kook M.-C., Seo M.-J., Cheigh C.-I., Pyun Y.-R., Cho S.-C., Park H. Enhanced production of gamma-aminobutyric acid using rice bran extracts by Lactobacillus sakei B2-16. J. Microbiol. Biotechnol. 2010;20(4):763–766. [PubMed] [Google Scholar]
- 47.Caraceni T., Girotti F., Giovannini P., Pederzoli M., Parati E. Effects of DA agonist in Huntington disease hyperkinesia. Ital. J. Neurol. Sci. 1979;1(2):155–161. doi: 10.1007/BF02335845. [DOI] [PubMed] [Google Scholar]
- 48.Cepeda C., Murphy K., Parent M., Levine M.S. The role of dopamine in huntington's disease. Prog. Brain Res. 2013;211:235–254. doi: 10.1016/B978-0-444-63425-2.00010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]