Abstract
Distal common bile duct carcinoma (DCBDC) is a poorly characterized entity, for reasons such as variable terminology and difficulty in determining site of origin of intrapancreatic lesions. We compared clinicopathologic features of pancreatobiliary-type adenocarcinomas within the pancreas but arising from the distal common bile duct (CBD) (defined as tumors centered around and circumferentially involving the intrapancreatic CBD) with those of pancreatic and ampullary origin. Upon careful review of 1017 pancreatoduodenectomy specimens with primary adenocarcinoma, 52 (5.1%) qualified as intrapancreatic DCBDC. Five associated with an intraductal papillary neoplasm were excluded; the remaining 47 were compared to 109 pancreatic ductal adenocarcinomas (PDAC) and 133 pancreatobiliary-type ampullary adenocarcinomas (AC). DCBDC patients had a younger median age (58 years) than PDAC patients (65 years) and AC patients (68 years). DCBDC was intermediate between PDAC and AC with regard to tumor size and rates of lymph node metastases and margin positivity. Median survival was better than for PDAC (P=0.0010) but worse than for AC (P=0.0006). Microscopically, DCBDC often formed an even band around the CBD and commonly showed intraglandular neutrophil-rich debris and a small tubular pattern. Poor prognostic indicators included lymph node metastasis (P=0.0010), lymphovascular invasion (P=0.0299), and margin positivity (P=0.0069). Categorizing the tumors based on size also had prognostic relevance (P=0.0096), unlike categorization based on anatomic structures invaded (P=0.1575). Primary DCBDC is seen in younger patients than PDAC or AC and has a prognosis significantly better than PDAC and worse than AC, at least partly due to differences in clinical presentation. Use of size-based criteria for staging appears to improve its prognostic relevance. When identified through careful dissection of pancreatoduodenectomy specimens, invasive pancreatobiliary-type DCBDC are uncommon in the West (5% of primary carcinomas in pancreatodudoenectomies) and have substantial clinicopathologic differences from their counterparts arising from the pancreas and ampulla.
Keywords: Cholangiocarcinoma, Extrahepatic Biliary Cancer, Pancreatic Cancer, Ampullary Cancer, Prognostic Factors
Cholangiocarcinoma, which accounts for approximately 3% of all gastrointestinal cancers, has an incidence of approximately 5,000 new cases per year in the United States,1 though incidence per capita is several times higher in many Eastern countries.2,3 While the World Health Organization uses the term “cholangiocarcinoma” only to refer to intrahepatic malignancies of biliary epithelial origin,4 other authors have used the term to encompass extrahepatic bile duct adenocarcinomas as well.5 These extrahepatic tumors have traditionally been divided into upper, middle, and lower categories,5 though considering that middle bile duct tumors are rare and are often treated the same as either upper or lower tumors, some authors have advocated eliminating the category and designating cholangiocarcinomas as either intrahepatic, perihilar, or distal.6,7 Regarding extrahepatic lesions, perihilar/upper are the most common, followed by distal, with the nebulous middle lesions the least common.6,8,9 While proximal and perihilar cholangiocarcinomas may be amenable to hepatic resection, surgically treatable distal tumors require pancreatoduodenectomy.
Although carcinomas of extrahepatic bile ducts are well analyzed in the East, their frequency and clinicopathologic associations in Western countries have been less thoroughly analyzed, particularly in the pathology literature. For example, the reported frequency of bile duct origin among what are called “periampullary carcinomas” (mostly referring to carcinomas removable by pancreatoduodenectomy) ranges from 5–17%,10–12 and this variation is most likely due to definitional differences. Moreover, distinguishing intrapancreatic distal common bile duct carcinoma (DCBDC) from pancreatic ductal adenocarcinoma (PDAC) and ampullary adenocarcinoma (AC) is often a challenge both clinically and pathologically, as there is significant anatomic and histologic overlap. Furthermore, DCBDC can be subtle on gross examination and can secondarily involve the main pancreatic duct and/or the ampulla.13 Additionally, secondary involvement of the common bile duct (CBD) is very common in PDAC; in our experience, 97.2% of PDACs show invasion into the intrapancreatic CBD.14 As a result, there has unsurprisingly been variation in the clinicopathologic characteristics of these entities reported in different studies, and many cases of these three carcinomas may have been incorrectly classified.
To address these issues, we performed a detailed pathologic review of 1017 pancreatoduodenectomy specimens with invasive adenocarcinoma by using more refined criteria in the classification of these tumors,15 along with a comparative clinicopathologic analysis distinguishing intrapancreatic DCBDC cases from PDAC and AC.
Materials and Methods
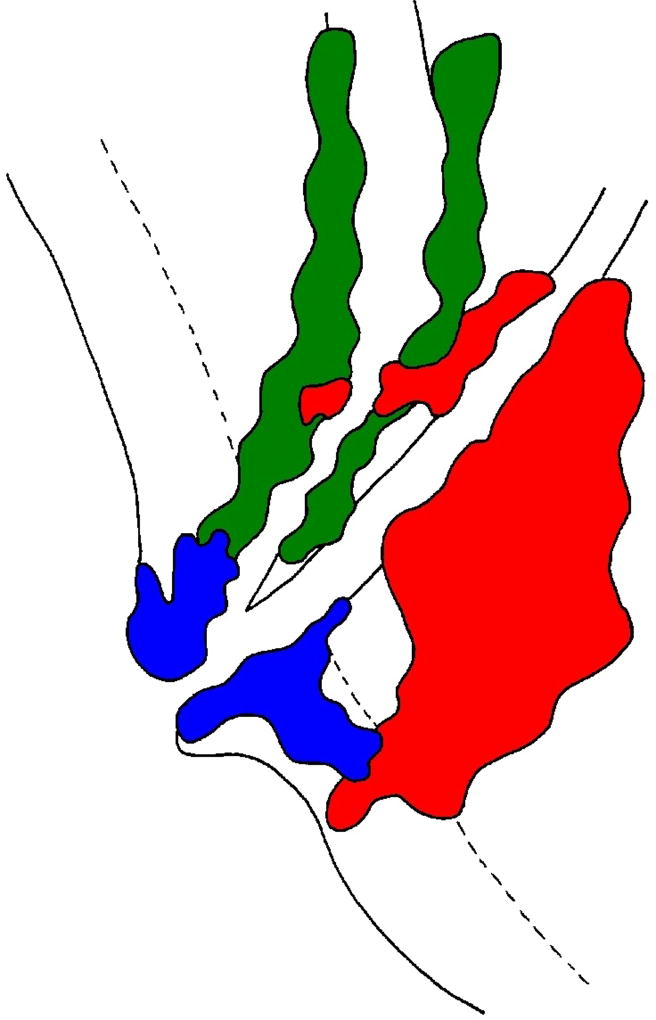
With appropriate Institutional Review Board approval, we performed a review of 1017 pancreatoduodenectomy specimens with the primary diagnosis of invasive adenocarcinoma, all resected in the United States; these specimens had been previously compiled into a computerized archive. Clinical history, gross photographs, available slides, and original signout report were all used to classify the tumors, with the original diagnoses overturned in some instances. During this process, DCBDC was defined as a carcinoma either grossly or microscopically centered unequivocally around the intrapancreatic CBD (Figure 1). Cases where the site of origin was not entirely clear, but was most likely the CBD (e.g., more than 75% of the tumor oriented around the bile duct) were also classified as DCBDC. Dubious cases (e.g., tumor halfway involving the pancreatic duct and halfway involving the DCBDC, or tumors causing biliary-type symptoms clinically but primarily sparing the CBD grossly) were excluded. The exact number of diagnoses changed during this process was not recorded.
Figure 1.
Pancreatic ductal adenocarcinoma (PDAC, red), ampullary carcinoma (AC, blue), and distal common bile duct carcinoma (DCBDC, green) all arise within close proximity to one another and can therefore overlap in terms of the anatomic space they occupy. Furthermore, PDAC can secondarily involve the ampulla or common bile duct, and AC and DCBDC can similarly invade structures from which they did not originate. Careful consideration of the size, epicenter, presentation, and histology of each lesion should allow for accurate diagnosis.
Fifty-two cases (5.1%) met inclusion criteria. Five cases associated with an intraductal papillary neoplasm of the bile duct (IPNB; the biliary counterpart of pancreatic intraductal papillary mucinous neoplasm [IPMN])4,16–18 were excluded to eliminate potentially confounding factors; all five were conventional adenocarcinomas and were from four men and one woman. The remaining 47 cases came from Emory University, Atlanta, GA (33 cases); Wayne State University, Detroit, MI (13 cases); and Piedmont Hospital, Atlanta, GA (1 case). Slides from these cases were reviewed by three of the authors (RSG, PB, NVA). The morphology of each tumor was scrutinized, with an emphasis on various patterns of growth and differentiation, as well as factors that might distinguish them from PDAC or AC. Biliary intraepithelial neoplasia (BilIN) was noted when present, and anomalous anatomy of the biliary tree was documented if identified. Margin and node status, presence or absence of lymphovascular and perineural invasion, and tumor stage were all reappraised.
Appropriate data for comparative analysis, including patient survival, was available for a separate cohort of 109 PDAC in our database. All cases, which were removed by pancreatoduodenectomy in the United States, were conventional pancreatobiliary-type adenocarcinomas arising within the pancreas. To eliminate confounding factors, colloid-type and IPMN-associated cases were excluded from the analysis, as these have been shown to be different biologically from ordinary pancreatobiliary-type adenocarcinomas.19,20 Malignancies other than adenocarcinoma were also excluded. Appropriate data for comparative analysis was also available for a separate cohort of 133 AC with pancreatobiliary-type morphology in our database. Only cases with predominantly pancreatobiliary-type histology were included in this group, in order to have a more fair and uniform comparison with PDAC and DCBDC.
The following clinicopathologic parameters were compared among the DCBDC, PDAC, and AC groups: patient age and sex, tumor size, presence of positive surgical margins and lymph node metastases, and clinical outcome. Data on distant metastases was missing from a majority of PDAC cases, precluding the use of this variable. Cases were not controlled for post-operative therapy, but there were no particular bias toward a specific treatment protocol based on the classification. Differences among the groups were assessed by ANOVA (patient age and tumor size), chi-square (patient sex, margin status, and metastases), and log-rank (survival) tests. Multivariable regression was conducted using Cox proportional hazard models to compare the predicted survival among the three tumor groups, independent of other factors. The proportional hazard assumption was tested for all variables using log-log survival curves. The final model was created using backward elimination to retain only the variables that were statistically significant predictors of survival.
Within the DCBDC group, independent factors potentially associated with survival, including tumor size, margin and node status, perineural and lymphovascular invasion, and American Joint Committee on Cancer (AJCC) T-classification and stage,21 were analyzed using Wald tests from unadjusted Cox models. To compare the predictive utility of certain factors, the Schwarz Bayesian Criteria (SBC) and C-index were calculated as measures of goodness of fit and discriminatory power. Lymph node harvesting results using two different methods were compared using an unpaired t test. All tests were two-sided, and statistical significance was defined as P-value <0.05. Statistical analyses were performed using SAS v 9.3 (SAS Institute, Cary, NC).
Results
Table 1 shows clinicopathologic comparisons among the DCBDC, PDAC, and AC groups.
TABLE 1.
Clinicopathologic comparison of pancreatic ductal adenocarcinoma (PDAC), distal common bile duct carcinoma (DCBDC), and ampullary adenocarcinoma (AC).
| PDAC (n=109) |
DCBDC (n=47) |
AC (n=133) | P-value1 | P-value2 | |
|---|---|---|---|---|---|
| Median age, years | 65 | 58 | 68 | 0.0576 | 0.0018 |
| Sex | 0.1612 | 0.4467 | |||
| Male | 47 (43.1%) | 26 (55.3%) | 63 (48.8%) | ||
| Female | 62 (56.9%) | 21 (44.7%) | 66 (51.2%) | ||
| Median tumor size, cm | 3.0 | 2.5 | 1.8 | 0.0105 | 0.0002 |
| Lymph node status | 0.0902 | 0.0915 | |||
| Negative | 27 (25.5%) | 18 (39.1%) | 64 (53.8%) | ||
| Positive | 79 (74.5%) | 28 (60.9%) | 55 (46.2%) | ||
| Margin status | 0.0339 | 0.0005 | |||
| Negative | 54 (58.7%) | 34 (77.3%) | 124 (96.1%) | ||
| Positive | 38 (41.3%) | 10 (22.7%) | 5 (3.9%) | ||
| Survival rates | 0.0010 | 0.0006 | |||
| 1-year | 44% | 82% | 86% | ||
| 3-year | 12% | 29% | 58% | ||
| 5-year | 7% | 20% | 39% | ||
| Median survival, months | 11 | 19 | 40 |
P-values comparing DCBDC and PDAC
P-values comparing DCBDC and AC
Note: Some cases of each tumor type had points of data missing; these were excluded from analysis in the relevant category.
Clinical Findings in DCBDC
The 47 DCBDC patients included 26 men and 21 women, with a median age at resection of 58 years (range 39–80). This was younger than the PDAC patients (median age 65; P=0.0576) and significantly younger than the AC patients (median age 68; P=0.0018).
Clinical history was available for review on 70.2% of the patients (33/47). The most common clinical symptom was jaundice, with 27/33 patients initially presenting with it (81.8%) and three more developing it during their clinical course. Other symptoms indicating biliary obstruction, such as scleral icterus, pruritis, dark urine, and light stools, were reported by 14 patients (42.4%), including two of the patients who did not develop jaundice. Other common symptoms included abdominal pain (14/33, 42.4%), weight loss (13/33, 39.4%), diarrhea (5/33, 15.2%), nausea (5/33, 15.2%), and vomiting (4/33, 12.1%). Imaging was available for 68.1% of the patients (32/47) and depicted a stricture of the distal biliary system in half (16/32, 50%). The other patients were interpreted as having a biliary mass (10/32, 31%) or a pancreatic mass (6/32, 19%); the 6 with a “pancreatic mass” only underwent computed tomography (CT). The combined clinical and imaging findings led to the correct diagnosis preoperatively in all 33 patients. None of the cases was known to have any association with biliary parasites or demonstrable pancreatobiliary duct malunion, and only one had a documented choledochal cyst.
Pathologic Findings in DCBDC
The DCBDC had a median size of 2.5 cm (range: 1.0–5.8 cm), a lymph node metastasis rate of 60.9%, and a positive margin rate of 22.7%. The retroperitoneal margin was the most frequently involved (6/44, 13.6%), with the CBD (3/44, 6.8%) and vascular bed (1/44, 2.3%) margins sometimes involved. As with prognosis (see below), all three of these figures placed DCBDC directly in between PDAC and AC; these relationships were statistically significant for median size and margin positivity rate, but not quite for lymph node metastasis rate (see Table 1). Lymphovascular invasion was observed in 40.4% of cases.
The median number of lymph nodes examined per case was 16. A specific form of lymph node harvesting (the “orange peeling” approach)22 had been employed in 61.7% of the cases, and the median number of nodes identified by this method was 19, vs. 6 in cases not “orange-peeled” (performed before this approach had been instituted) (P<0.0001). The median number of involved nodes in node-positive cases was 3. Thirteen patients (27.7%) had documented distant metastases, with the liver the most commonly involved site.
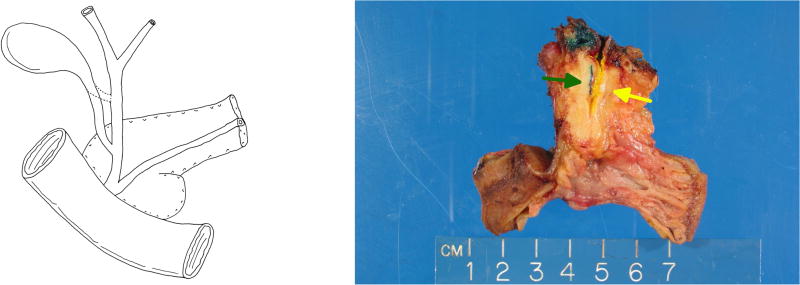
Four cases showed cystic duct–CBD union within the pancreas (“low union”), with the tumor immediately distal to this abnormal junction (Figures 2a and 2b); this anomaly was observed in one man and three women.
Figure 2.
(a) In four of our DCBDC cases, the common hepatic duct and cystic bile duct did not join until both were within the pancreas, creating a short and entirely intrapancreatic common bile duct. We refer to this phenomenon as “low union” of the ducts. (b) A pancreatoduodenectomy specimen demonstrating low union. Green ink and green arrow denote cystic duct; yellow ink and yellow arrow denote common hepatic duct.
Most DCBDC (41/47; 87.2%) were pT3 by the current AJCC criteria (7th edition) at resection, with 3/47 (6.4%) being pT1 and 3/47 being pT2. No T-classification or other staging comparison was performed between DCBDC and the PDAC or AC cases, as the staging parameters used for these three sites are vastly different, are mostly arbitrary, present significant challenges in their application, and are undergoing revision.14,23–30
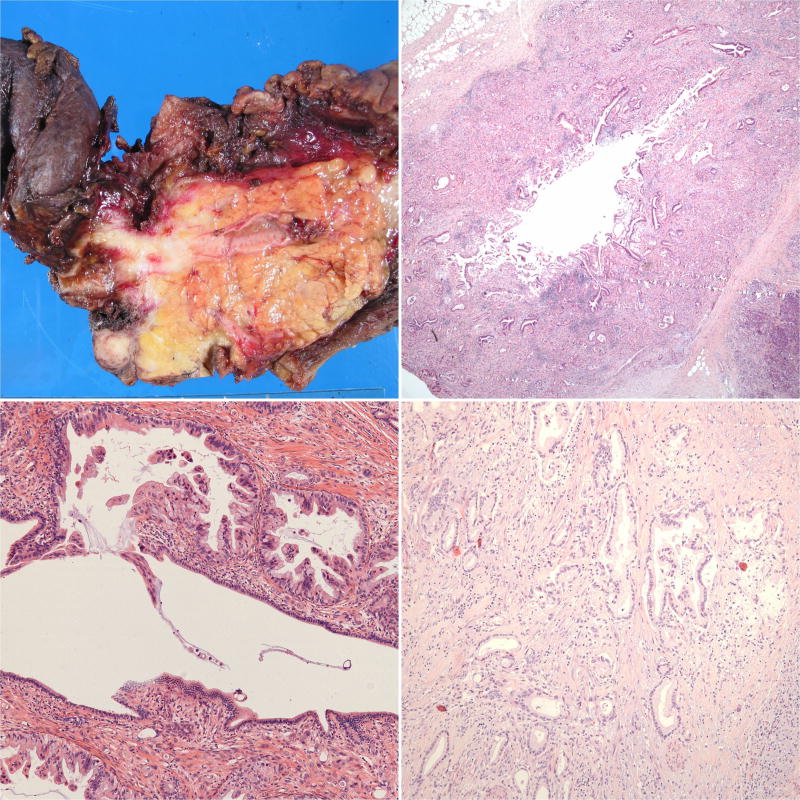
Given the retrospective nature of the study and the fact that the 47 DCBDC came from multiple different institutions, gross approach to the pancreatoduodenectomy specimens was not uniform. Still, valuable information could be gleaned from most of the gross descriptions: DCBDC were often subtle, scirrhous, constrictive lesions forming circumferential plaque-like thickening of the CBD wall in a lunar or semilunar pattern (Figure 3a). In some cases, however, evidence of malignancy was limited to friability and erythema of the duct mucosa. Gross examination failed to detect subtle carcinoma in a few cases.
Figure 3.
(a) This pancreatoduodenectomy specimen demonstrates a distal common bile duct carcinoma forming a constrictive lesion that surrounds and tracks along the common bile duct within the pancreas. It also extends partially into the main pancreatic duct and the ampulla; however, the tumor epicenter is at the common bile duct. (b) This pattern of spread can also be observed microscopically. (c) This carcinoma instead traverses just underneath the common bile duct surface, occasionally penetrating it. Intraepithelial neoplasia is not present in this portion of the duct. (d) Distal common bile duct carcinoma morphologically resembles typical pancreatobiliary-type adenocarcinomas in most cases.
Microscopically, DCBDC often formed an even band around the CBD (Figure 3b), which could be particularly striking in the pancreas-neighboring aspects of the duct. Careful analysis in these situations revealed more infiltrative foci, especially in the posterior aspect of the CBD, where soft tissue is immediately adjacent. In two cases, the carcinoma tracked along the CBD microscopically, traveling just underneath the epithelium but occasionally penetrating into the main duct lumen (Figure 3c). The tumor often secondarily involved pancreatic ducts and mimicked pancreatic intraepithelial neoplasia (PanIN)-3, underscoring the need for careful gross examination in establishing the precise site of tumor origin.
The infiltrative glands in DCBDC closely resembled those of PDAC, namely one or two layers of cuboidal cells forming small lumens (Figure 3d). In cases of well-differentiated carcinoma, infiltration was at times subtle and hard to distinguish from surrounding normal duct structures. Perineural invasion was a common finding (91.5%), as it is in PDAC.
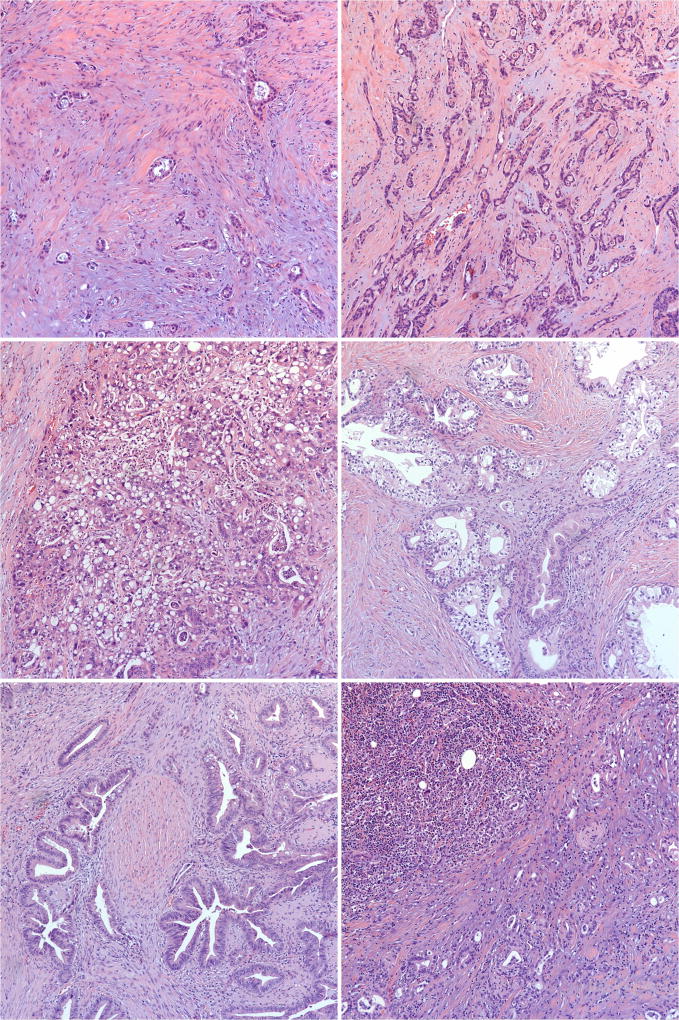
Compared to typical PDAC morphology, DCBDC more commonly showed intraglandular neutrophil-rich debris and a smaller tubular pattern. Other histologic patterns focally observed in otherwise pancreatobiliary-type DCBDC included a tubulolobular pattern akin to that of invasive lobular carcinoma of the breast (2/47 cases, 4.3%; Figure 4a); a microglandular pattern reminiscent of mesonephric remnants (7/47, 14.9%; Figure 4b); a vacuolated cell pattern, as documented recently in PDAC31 (2/47, 4.3%; Figure 4c); a clear cell or foamy gland appearance (2/47, 4.3%; Figure 4d); an intestinal-like pattern that demonstrated goblet cells (3/47, 6.4%; Figure 4e); medullary morphology (1/47, 2.1%; Figure 4f); and prominent papilla formation within the invasive glands (1/47, 2.1%).
Figure 4.
Morphologic variants of otherwise pancreatobiliary-type distal common bile duct carcinoma. (a) Tubulolobular features, reminiscent of mammary carcinoma. (b) Microglandular features with intraluminal acidophilic secretions (“mesonephric” pattern). (c) Vacuolated features. (d) Clear-cell and foamy gland features. (e) Intestinal-type features. (f) Medullary features.
The CBD mucosa showed what could be interpreted as high-grade biliary intraepithelial neoplasia (BilIN-3)32 in 28 cases (59.6%), though it was impossible to determine whether these lesions represented true BilIN or duct cancerization. This finding was often present only focally, though large portions of the CBD epithelium were denuded in virtually every specimen (presumably secondary to stenting and/or manipulation of the gross specimen), potentially removing evidence of an intraepithelial component in some cases. While BilIN was mostly of the simple, flat type, 5 cases (10.6%) demonstrated a micropapillary pattern (Figure 5a), and in one case, it took on an intestinal appearance, mimicking low- and high-grade dysplasia in a colonic tubular adenoma (Figure 5b). One case demonstrated multifocal BilIN along the entire intrapancreatic CBD.
Figure 5.
(a) High-grade biliary intraepithelial neoplasia (BilIN) growing in a micropapillary pattern. (b) BilIN simulating intestinal-type low- and high-grade dysplasia.
Incidental findings noted during analysis of the DCBDC cases included several foci of PanIN. Most foci were PanIN-1 and -2, which are known to be common incidental findings in pancreata,33 though two patients had small foci of severely atypical cells well away from the primary tumor, raising the differential of PanIN-3 versus spread of the carcinoma to the pancreatic ducts. Other incidental observations, each seen o, included pancreas divisum, squamoid cyst of the pancreatic duct, duodenal lymphangioma, duodenal well-differentiated neuroendocrine tumor, and traumatic neuroma adjacent to and involved by the DCBDC.
Outcome
Factors associated with worse clinical outcome in DCDBC included tumor size (P=0.0402), lymph node metastases (P=0.0010), lymphovascular invasion (P=0.0299), and margin positivity (P=0.0069). Perineural invasion did not affect patient survival (P=0.2204) but was only absent in four cases.
Grouping the DCBDC according to AJCC T-classification was not significantly related to survival (P=0.1575). However, stratifying the DCBDC into three groups based on maximum tumor dimension (size < 2 cm, size 2–4 cm, and size > 4 cm) was strongly associated with survival, as patients with larger tumors fared worse (P=0.0096). This result held when comparing the two groups using the SBC, which is a type of information criteria used in model selection as a measure of goodness of fit; the SBC was 186.23 for AJCC T-classification and 183.73 for size stratification, with a lower SBC indicating a better fit of the model. However, when using the C-index, which is a measure of model discrimination created as an extension of the receiver operating characteristic that can be applied to survival data, AJCC T-classification was a better fit than size stratification (0.8389 and 0.8149, respectively, with a higher C-index indicating better predictive ability). Therefore, two of three comparisons favored size stratification over AJCC T-classification.
In a similar vein, a “modified AJCC” system, where the three aforementioned size cutoffs were used in place of the AJCC definitions for pT1/pT2/pT3, better predicted patient outcome than non-modified AJCC staging. The modified system had a P-value of 0.0030, an SBC of 188.06, and a C-index of 0.7145, all of which were more favorable than the non-modified AJCC system (P-value of 0.0086, SBC of 191.04, C-index of 0.7023).
Follow-up data was available for 45 DCBDC patients (median: 18 months; range: 0–157 months). Seven were alive at last follow-up (median: 36 months). One was still alive 114 months after surgery, and two of the deceased survived for 157 and 104 months; such survival lengths are almost unheard of in PDAC.34
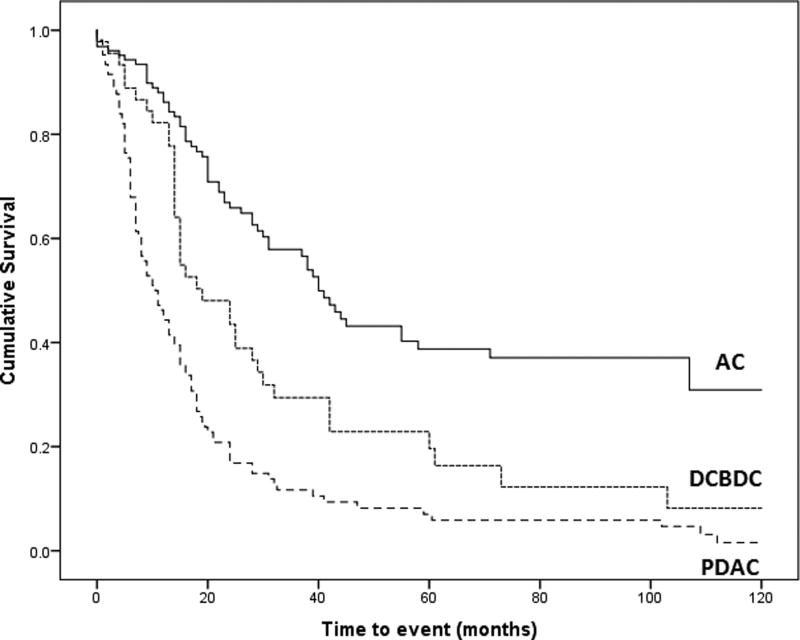
The 1-year, 3-year, 5-year, median survival, and log-rank test all showed increased survival for DCBDC patients compared with PDAC patients (P=0.0010) and worse survival compared with AC patients (P=0.0006) (Figure 6). In the multivariable analysis including all three groups, factors significantly impacting survival included older age (P=0.0107), margin involvement (P=0.0008), and nodal disease (P<0.0001). These characteristics were retained in the final model. Using this adjusted model and patients with AC tumors as the reference group, the hazard ratio for patients with DCBDC was 2.3 (P=0.0005), and the hazard ratio for patients with PDAC was slightly lower, at 2.1 (P=0.0041).
Figure 6.
Kaplan–Meier survival curves for ampullary adenocarcinoma (AC), distal common bile duct carcinoma (DCBDC), and pancreatic ductal adenocarcinoma (PDAC). Patients with DCBDC fared worse than patients with AC (P=0.0006) but better than patients with PDAC (P=0.0010, log-rank test).
Discussion
There have been several impediments in the literature in characterizing the clinicopathologic features of DCBDC. Classification and terminology of cholangiocarcinomas have varied over time; currently, most accepted schemes use “distal” CBD carcinoma synonymously with “intrapancreatic cholangiocarcinoma,” though tumors arising outside the pancreas but within the distal extrapancreatic CBD are considered DCBDC as well and usually are surgically approached with pancreatoduodenectomy.6 Overall, DCBDC appears to be the most straightforward and encompassing term for these tumors. The argument over whether these extrahepatic tumors are “cholangiocarcinomas” or “adenocarcinomas” is beyond the scope of this project, hence the use of “distal CBD carcinoma” for our cohort of cases removed by pancreatoduodenectomy.
While cholangiocarcinoma overall is not an especially rare diagnosis in Western countries, it appears far more prevalent in Eastern countries, mostly attributable to association with parasites.2,3 Accordingly, many studies dealing with the clinicopathologic aspects of DCBDC have been from Japan,8,35–40 Korea,41 and China.9 Recently, certain groups in the United States have described and analyzed series of this entity as well.7,10,42,43 However, many of these studies lumped DCBDC in with proximal cases and/or did not include pathologic review, meaning they may well have included cases of PDAC and AC masquerading as DCBDC. Our rigorous efforts to exclude intrapancreatic cancers of non-biliary origin may explain why only 5.1% of the adenocarcinomas in our pancreatoduodenectomies were DCBDC, whereas other studies have found rates of 12%10 to 17%.11
Known clinical risk factors for “cholangiocarcinoma” include parasites, primary sclerosing cholangitis, choledochal cysts, Thorotrast exposure, viral hepatitis, and possibly choledocholithiasis.3,40,44 Our study observed a previously unrecognized factor that may also be important in the pathogenesis of DCDBC: union of the cystic duct and common hepatic duct within the pancreas. In these cases, the cystic duct, rather than joining with the common hepatic duct just distal to the hepatic hilum, traveled separately toward the pancreas, entered it, and joined the common hepatic duct there, creating a short, entirely intrapancreatic CBD. While several anomalous configurations of the biliary tree have been described,5 this appears to be a relatively unexplored phenomenon that we have referred to as “low union” of the extrahepatic biliary system.45 As biliary carcinoma can arise in anatomic abnormalities such as pancreatobiliary maljunction46 and bile duct duplication,47 we suspect that low union can contribute to the development of intrapancreatic malignancy.45 The incidence of low union in the general population is unknown. As more careful grossing protocols are adopted,48 its true incidence and potential role in tumorigenesis may become better appreciated.
Clinically, as demonstrated in this study, many DCBDC patients present with symptoms related to biliary obstruction; as a result, they tend to present earlier than patients who have an intrahepatic biliary lesion.44 However, intrapancreatic DCBDC may present with more stereotypically pancreatic symptoms, such as pain, abdominal fullness, early satiety, and weight loss.4,44,49
Radiologic techniques, such as CT, magnetic resonance imaging, and endoscopic retrograde cholangiopancreatography, can help determine the site of origin of an intrapancreatic lesion.5,50 However, pancreatic head lesions cannot always be localized correctly preoperatively.5 In our study, proper synthesis of clinical and imaging data allowed for a diagnosis of DCBDC to be made prior to surgery in each case.
For cases where the preoperative diagnosis is unclear or in question, careful gross examination of the pancreatoduodenectomy specimen is essential, and should be standardized as much as possible.48 The epicenter of the gross lesion should be considered, as well as what structures are involved and in what fashion. Some DCBDC are obviously distinct from PDAC, as they involve the CBD in a circumferential fashion, forming a constrictive lesion that spreads along the duct lengthwise. Less overt cases can be challenging, especially considering that PDAC secondarily invades the CBD in the vast majority of cases.14 However, it would be unusual in our experience for PDAC to involve the CBD circumferentially rather than in a haphazard, disorganized fashion. Accordingly, a recent study reported that intrapancreatic tumors symmetrically/concentrically involving the CBD are likely to be DCBDC, whereas asymmetric/eccentric involvement generally implies a PDAC.42 Large tumors that destroy all the surrounding anatomic structures are also most likely to be PDAC, as it is the more common tumor and the more likely to be large in size at time of resection, primarily because it does not cause jaundice as readily as DCBDC. Nebulous tumors that could have possibly arisen from one of several structures based on their anatomic location (for example, those with the epicenter right at the junction of the CBD and ampulla) require methodical microscopic analysis of the ductal system for intraepithelial neoplasia, as well as careful correlation with clinical and radiologic findings. Furthermore, PDAC and DCBDC are similar histologically; while our study found that intraluminal neutrophils were often seen in DCBDC, they can occasionally be seen in PDAC as well.51,52
In some instances, unfortunately, it may well be impossible to classify an intrapancreatic tumor correctly, calling the prognosis of the patient and the stage of the tumor into question. We found that DCBDC has significantly longer survival than PDAC and significantly shorter survival than AC. Other studies have reached similar conclusions regarding the relative prognosis of these three tumors, and many found that tumor type is an independent prognostic indicator on multivariable analysis.10–12,39 It should be noted that, unlike other studies, we have included only carcinomas of pancreatobiliary-type morphology in our cohorts, and we have also excluded adenocarcinomas arising from IPMN or IPNB. Comparison of these purified categories disclosed a potentially important facet of these carcinomas: On multivariable analysis, we found that PDAC has a hazard ratio of 2.1 compared to AC, while DCBDC has a hazard ratio of 2.3. This suggests that DCBDC is intrinsically as aggressive as PDAC, if not more so. This is not necessarily surprising, as DCBDC and PDAC are both conventional pancreatobiliary-type adenocarcinomas and are very similar to each other. DCBDC likely has an overall better prognosis because it manifests relatively early (due to CBD obstruction) and is therefore discovered at a lower stage. Still, the higher hazard ratio of DCBDC may be related to either a more aggressive inherent biology or to the fact that DCBDC has ready access to the peritoneal cavity and may spread more easily than recognized by clinical or pathologic evaluation.
Criteria for tumor staging also differ among the three entities. At the time of this writing, the AJCC Cancer Staging Manual is on its 7th Edition. This edition, in addition to a classification for pancreas (endocrine and exocrine) lesions, has a separate classification for distal bile duct tumors,21 whereas the 6th Edition included distal bile duct lesions in its chapter on all extrahepatic bile duct tumors. In the 7th Edition, invasion of the pancreas by DCBDC is classified as pT3, whereas invasion of only the pancreas in PDAC is pT1 or pT2, depending on size. Hong et al.29,30 have taken issue with the AJCC’s approach of staging distal biliary malignancy by involvement of nearby structures. They note that the anatomy of the extrahepatic bile duct wall is variable throughout its length, making distinction between pT1 tumors (confined to the bile duct) and pT2 tumors (invading beyond the bile duct wall but not into adjacent organs) difficult, if not impossible. They also found no survival difference between AJCC pT2 and pT3 carcinomas. Instead, they proposed a classification based entirely on depth of invasion, with 5 mm and 12 mm of spread from the CBD epithelial surface offered as cutoff points with statistically significant differences in prognosis.29,30 As the DCBDC cases in our study were not grossed in a uniform fashion, we were unable to perform the same careful, goal-directed analysis that Hong’s group did. Still, we similarly found that the current AJCC T-classification did not correlate with prognosis. Instead, we found that size-based criteria – specifically, stratifying DCBDC cases by greatest dimension (< 2 cm, 2–4 cm, > 4 cm), as is now being proposed for PDAC25 – correlated significantly with prognosis, both in isolation and as a substitute for AJCC T-classification. These results, in conjunction with Hong’s, indicate that a size-based T-classification system is appropriate for staging DCBDC. In any case, the size of an invasive carcinoma should be documented in the surgical pathology report and taken into consideration in assessing the prognosis of a given case. Of note, some surgical studies have reported that size is not an independent prognostic factor, but those studies analyzed extrahepatic bile duct tumors as a whole.7–9
In addition to tumor size, we found that lymphovascular invasion and nodal metastases significantly impact prognosis in DCBDC. The lymphatic system supplying the distal biliary tree drains to lymph nodes around the head of the pancreas, making harvesting of the nodes in a pancreatoduodenectomy specimen appropriate for DCBDC.53 Many of the aforementioned studies also found that node status7–9,35–38,41–43 is a key prognostic indicator in DCBDC patients. Some also reported that prognosis worsens with increased number of positive nodes.35–37,41
The prognostic significance of nodal disease underscores the importance of accurate gross sampling of lymph nodes, with examination of at least 12 nodes recommended for pancreatoduodenectomy specimens.54 We employ an “orange-peeling” technique22,48 that has been proven to increase both node count and percentage of node-positive cases. This approach was used in 61.7% of our DCBDC cases in this study and more than tripled the median number of nodes found in each case.
Most studies on DCBDC, ours included, found positive margins to negatively impact prognosis,6,7,35,37,38 though a few did not.8,43 Perineural invasion, found by some8,9,35 but not all37,41 other groups to be a negative prognostic indicator, did not have a prognostic impact in our study, though our rate of perineural invasion (91.5%) was higher than in these other studies, presumably due to the more careful scrutiny we employed during re-review of the cases, along with the careful exclusion of non-pancreatobiliary-type cancers, which are less prone to show perineural invasion.
In summary, while less common than their intrahepatic counterparts, distal common bile duct carcinomas are a distinct entity that can require careful clinical, gross, and/or microscopic evaluation in order to properly identify. As they possess significantly different clinicopathologic characteristics than PDAC and AC, which they can approximate both morphologically and anatomically, due diligence is warranted in order to provide accurate prognostic information to patients. While factors such as smaller tumor size and lower rate of margin positivity in DCBDC compared to PDAC may be due to factors such as earlier onset of symptoms and increased ease of total resection due to tumor location, the relatively younger mean age of DCBDC patients may be related to unknown genetic factors, including those that give rise to ductal anomalies such as low union.
Footnotes
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–25. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 2.Shin HR, Oh JK, Masuyer E, et al. Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma--focus on East and South-Eastern Asia. Asian Pac J Cancer Prev. 2010;11:1159–66. [PubMed] [Google Scholar]
- 3.Nishimura M, Naka S, Hanazawa K, et al. Cholangiocarcinoma in the distal bile duct: a probable etiologic association with choledocholithiasis. Dig Dis Sci. 2005;50:2153–8. doi: 10.1007/s10620-005-3023-9. [DOI] [PubMed] [Google Scholar]
- 4.Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO Classification of Tumours of the Digestive System. Fourth. Lyon: IARC Press; 2010. [Google Scholar]
- 5.Blumgart LH, editor. Surgery of the liver, biliary tract, and pancreas. 4. Philadelphia: Saunders; 2006. [Google Scholar]
- 6.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhuiya MR, Nimura Y, Kamiya J, et al. Clinicopathologic factors influencing survival of patients with bile duct carcinoma: multivariate statistical analysis. World J Surg. 1993;17:653–7. doi: 10.1007/BF01659134. [DOI] [PubMed] [Google Scholar]
- 9.He P, Shi JS, Chen WK, et al. Multivariate statistical analysis of clinicopathologic factors influencing survival of patients with bile duct carcinoma. World J Gastroenterol. 2002;8:943–6. doi: 10.3748/wjg.v8.i5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Ahuja N, Makary MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford) 2014;16:83–90. doi: 10.1111/hpb.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–72. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt CM, Powell ES, Yiannoutsos CT, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:718–25. doi: 10.1001/archsurg.139.7.718. [DOI] [PubMed] [Google Scholar]
- 13.Shen J, Konstantinidis I, Ferrone CR, et al. Intrapancreatic Cholangiocarcinomas: A Clinicopathological and Immunohistochemical Analysis. Mod Pathol. 2010;23(Suppl 1s):371A. [Google Scholar]
- 14.Oliva IV, Bandyopadhyay S, Coban I, et al. Incidence and Significance of Common Bile Duct Involvement in Resected Pancreatic Ductal Adenocarcinomas: Should It Be Represented in the TNM Staging? Mod Pathol. 2009;22(Suppl 1s):319A. [Google Scholar]
- 15.Adsay V, Ohike N, Tajiri T, et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. 2012;36:1592–608. doi: 10.1097/PAS.0b013e31826399d8. [DOI] [PubMed] [Google Scholar]
- 16.Zen Y, Fujii T, Itatsu K, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–43. doi: 10.1002/hep.21387. [DOI] [PubMed] [Google Scholar]
- 17.Rocha FG, Lee H, Katabi N, et al. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352–60. doi: 10.1002/hep.25786. [DOI] [PubMed] [Google Scholar]
- 18.Barton JG, Barrett DA, Maricevich MA, et al. Intraductal papillary mucinous neoplasm of the biliary tract: a real disease? HPB (Oxford) 2009;11:684–91. doi: 10.1111/j.1477-2574.2009.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26–42. doi: 10.1097/00000478-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–95. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Byrd DR, Carducci MA, et al., editors. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2009. [Google Scholar]
- 22.Adsay NV, Basturk O, Altinel D, et al. The number of lymph nodes identified in a simple pancreatoduodenectomy specimen: comparison of conventional vs orange-peeling approach in pathologic assessment. Mod Pathol. 2009;22:107–12. doi: 10.1038/modpathol.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adsay NV, Bagci P, Tajiri T, et al. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 2012;29:127–41. doi: 10.1053/j.semdp.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Choi H, Saka B, Balci S, et al. Proposal for a Revised N-Stage for Pancreatic Ductal Adenocarcinoma as N1 (<3) and N2 (≥3) with Strong Prognostic Correlation. Mod Pathol. 2014;27(Suppl 2s):448A. [Google Scholar]
- 25.Saka B, Balci S, Basturk O, et al. Pancreatic Ductal Adenocarcinoma is Spread to the Peripancreatic Soft Tissue in the Majority of Resected Cases, Rendering the AJCC T-Stage Protocol (7th Edition) Inapplicable and Insignificant: A Size-Based Staging System (pT1: ≤2, pT2: >2-≤4, pT3: >4 cm) is More Valid and Clinically Relevant. Ann Surg Oncol. 2016 Jan 29; doi: 10.1245/s10434-016-5093-7. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saka B, Oliva I, Bandyopadhyay S, et al. Will the pT1 and pT2 Pancreas Cancer Please Stand Up? “Peripancreatic Soft Tissue” Is Involved in Most Pancreatic Ductal Adenocarcinomas (PDAC), Negating Its Value as a Staging Parameter and Necessitating a New Staging Scheme. Mod Pathol. 2014;27(Suppl 2s):454A. [Google Scholar]
- 27.Khayyata S, Thirabanjasak D, Basturk O, et al. Pitfalls In the Staging of Ampullary Carcinoma: Is The Current TNM (AJCC/UICC) System Pathologically And Clinically Relevant? Mod Pathol. 2007;20(Suppl 2s):119A. [Google Scholar]
- 28.Tajiri T, Ohike N, Balci S, et al. Proposal for a More Applicable and Clinically Relevant Staging Evaluation of Ampullary Carcinomas. Mod Pathol. 2011;24(Suppl 2s):169A. [Google Scholar]
- 29.Hong SM, Cho H, Moskaluk CA, et al. Measurement of the invasion depth of extrahepatic bile duct carcinoma: An alternative method overcoming the current T classification problems of the AJCC staging system. Am J Surg Pathol. 2007;31:199–206. doi: 10.1097/01.pas.0000213384.25042.86. [DOI] [PubMed] [Google Scholar]
- 30.Hong SM, Pawlik TM, Cho H, et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery. 2009;146:250–7. doi: 10.1016/j.surg.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dursun N, Feng J, Basturk O, et al. Vacuolated cell pattern of pancreatobiliary adenocarcinoma: a clinicopathological analysis of 24 cases of a poorly recognized distinctive morphologic variant important in the differential diagnosis. Virchows Arch. 2010;457:643–9. doi: 10.1007/s00428-010-0978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zen Y, Adsay NV, Bardadin K, et al. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2007;20:701–9. doi: 10.1038/modpathol.3800788. [DOI] [PubMed] [Google Scholar]
- 33.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16:996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- 34.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–9. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami Y, Uemura K, Hayashidani Y, et al. Pancreatoduodenectomy for distal cholangiocarcinoma: prognostic impact of lymph node metastasis. World J Surg. 2007;31:337–42. doi: 10.1007/s00268-006-0224-0. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki R, Takahashi M, Funato O, et al. Prognostic significance of lymph node involvement in middle and distal bile duct cancer. Surgery. 2001;129:677–83. doi: 10.1067/msy.2001.114555. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida T, Matsumoto T, Sasaki A, et al. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg. 2002;137:69–73. doi: 10.1001/archsurg.137.1.69. [DOI] [PubMed] [Google Scholar]
- 38.Kayahara M, Nagakawa T, Ohta T, et al. Role of nodal involvement and the periductal soft-tissue margin in middle and distal bile duct cancer. Ann Surg. 1999;229:76–83. doi: 10.1097/00000658-199901000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu Y, Kimura F, Shimizu H, et al. The morbidity, mortality, and prognostic factors for ampullary carcinoma and distal cholangiocarcinoma. Hepatogastroenterology. 2008;55:699–703. [PubMed] [Google Scholar]
- 40.Ito Y, Kenmochi T, Egawa T, et al. Diagnosis of Distal Cholangiocarcinoma after the Removal of Choledocholithiasis. Gastroenterol Res Pract. 2012:396869. doi: 10.1155/2012/396869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong SM, Cho H, Lee OJ, et al. The number of metastatic lymph nodes in extrahepatic bile duct carcinoma as a prognostic factor. Am J Surg Pathol. 2005;29:1177–83. doi: 10.1097/01.pas.0000160978.77833.d7. [DOI] [PubMed] [Google Scholar]
- 42.Deshpande V, Konstantinidis IT, Castillo CF, et al. Intra-pancreatic Distal Bile Duct Carcinoma is Morphologically, Genetically, and Clinically Distinct from Pancreatic Ductal Adenocarcinoma. J Gastrointest Surg. 2016;20:953–9. doi: 10.1007/s11605-016-3108-0. [DOI] [PubMed] [Google Scholar]
- 43.Fong Y, Blumgart LH, Lin E, et al. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996;83:1712–5. doi: 10.1002/bjs.1800831217. [DOI] [PubMed] [Google Scholar]
- 44.Veillette G, Castillo CF. Distal biliary malignancy. Surg Clin North Am. 2008;88:1429–47. xi. doi: 10.1016/j.suc.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez RS, Saka B, Bagci P, et al. Neoplasms and Pseudotumors Associated with Low (Intrapancreatic) Union of Cystic Duct into Common Bile Duct: A Clinicopathologic Analysis of 15 Cases of a Hitherto Unrecognized Phenomenon. Mod Pathol. 2013;26(Suppl 2s):424A. [Google Scholar]
- 46.Bragazzi MC, Cardinale V, Carpino G, et al. Cholangiocarcinoma: Epidemiology and risk factors. Transl Gastrointest Cancer. 2012;1:21–32. [Google Scholar]
- 47.Kosar I, Ataseven H, Yönem O, et al. A new variant of bile duct duplication with coexistence of distal cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2010;7:527–30. doi: 10.1038/nrgastro.2010.118. [DOI] [PubMed] [Google Scholar]
- 48.Adsay NV, Basturk O, Saka B, et al. Whipple made simple for surgical pathologists: orientation, dissection, and sampling of pancreaticoduodenectomy specimens for a more practical and accurate evaluation of pancreatic, distal common bile duct, and ampullary tumors. Am J Surg Pathol. 2014;38:480–93. doi: 10.1097/PAS.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiMagno EP. Pancreatic cancer: clinical presentation, pitfalls and early clues. Ann Oncol. 1999;10(Suppl 4):140–2. [PubMed] [Google Scholar]
- 50.Mangiavillano B, Mariani AA, Petrone MC. An intrapancreatic cholangiocarcinoma detected with optical coherence tomography during endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol. 2008;6:A30. doi: 10.1016/j.cgh.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Adsay NV, Bandyopadhyay S, Basturk O, et al. Chronic pancreatitis or pancreatic ductal adenocarcinoma? Semin Diagn Pathol. 2004;21:268–76. doi: 10.1053/j.semdp.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Smith RA, Tang J, Tudur-Smith C, et al. Meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Br J Cancer. 2011;104:1440–51. doi: 10.1038/bjc.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurosaki I, Tsukada K, Hatakeyama K, et al. The mode of lymphatic spread in carcinoma of the bile duct. Am J Surg. 1996;172:239–43. doi: 10.1016/S0002-9610(96)00156-0. [DOI] [PubMed] [Google Scholar]
- 54.Bilimoria KY, Bentrem DJ, Lillemoe KD, et al. Pancreatic Cancer Quality Indicator Development Expert Panel, American College of Surgeons. Assessment of pancreatic cancer care in the United States based on formally developed quality indicators. J Natl Cancer Inst. 2009;101:848–59. doi: 10.1093/jnci/djp107. [DOI] [PMC free article] [PubMed] [Google Scholar]