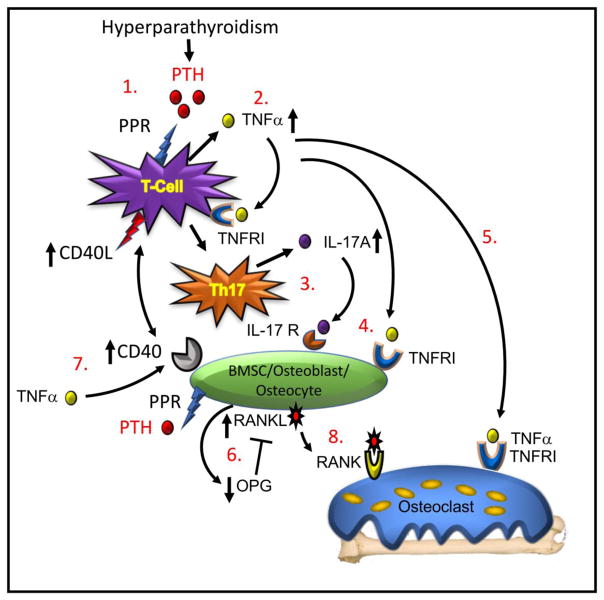
Figure 1. Model of T cell dependent mechanism of cPTH-induced bone loss.
(1.) Binding of PTH to its receptor (PPR) on the CD4+ T cell initiates (2.) production of TNFα, a cytokine that binds to a Type I receptor (TNFRI) and promotes differentiation of the T cell into (3.) a Th17 helper cell secreting IL-17A. (4.) Both of TNFα and IL-17A bind to receptors (TNFRI and IL-17R respectively) on BMSC, osteoblasts and osteocytes. (5.) TNFα further binds to osteoclastic cells amplifying RANKL activity on the differentiating osteoclast and stimulating bone resorption. (6.) TNFα and IL-17A promote production of RANKL and suppress production of OPG, the RANKL inhibitor, creating a balance of RANKL to OPG favourable to increased osteoclastic differentiation. (7.) TNFα further upregulates expression of CD40 on BMSC allowing a costimulatory signal to be transduced from the T cells though expression of CD40L. This signal primes the osteoblast to further respond to direct PTH stimulation to ensure robust RANKL production. (8) RANKL binds to its receptor RANK on osteoclast lineage cells causing osteoclast precursor differentiation and fusion into mature osteoclasts that resorb bone leading to bone loss. Model adapted from Li et al., [39].