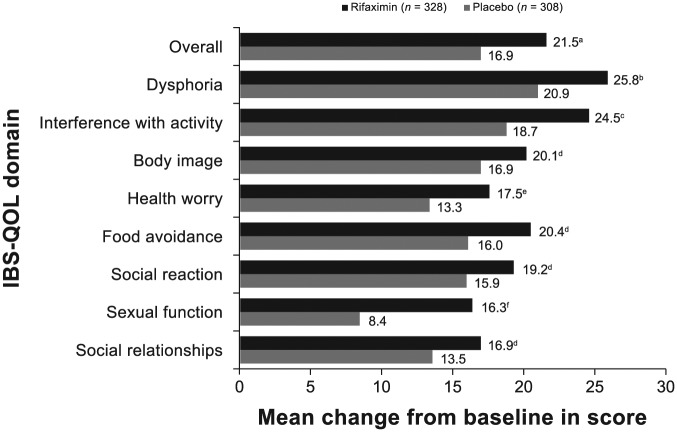
Figure 4.
Change from open-label baseline in IBS-QOL overall and subdomain scores at last visit for patients randomly assigned to receive rifaximin (n = 328) or placebo (n = 308) in the double-blind treatment phases. The p-value was based on a one-way ANOVA, with a factor of treatment group, adjusted for analysis center, time to recurrence, and recurrence type. Positive numbers indicate an improvement from baseline in IBS-QOL score. Number of patients with missing data: rifaximin (n = 2, overall score, dysphoria, interference with activity, body image, and food avoidance; n = 1, health worry, social reaction, sexual function, and social relationships); placebo (n = 1, overall and all subdomains).
a, p = 0.01; b, p = 0.02; c, p = 0.006; d, NS; e, p = 0.03; f, p < 0.001.
ANOVA, analysis of variance; IBS-QOL, irritable bowel syndrome quality of life questionnaire; NS, not significant.